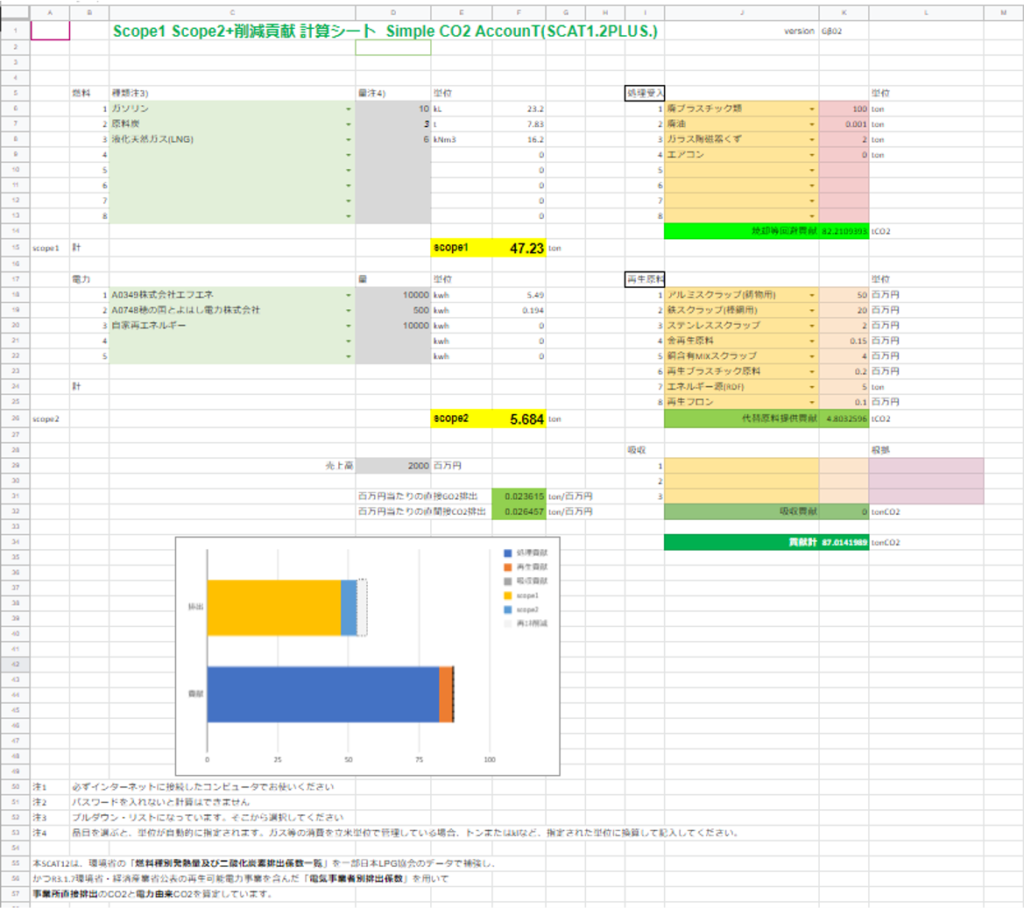
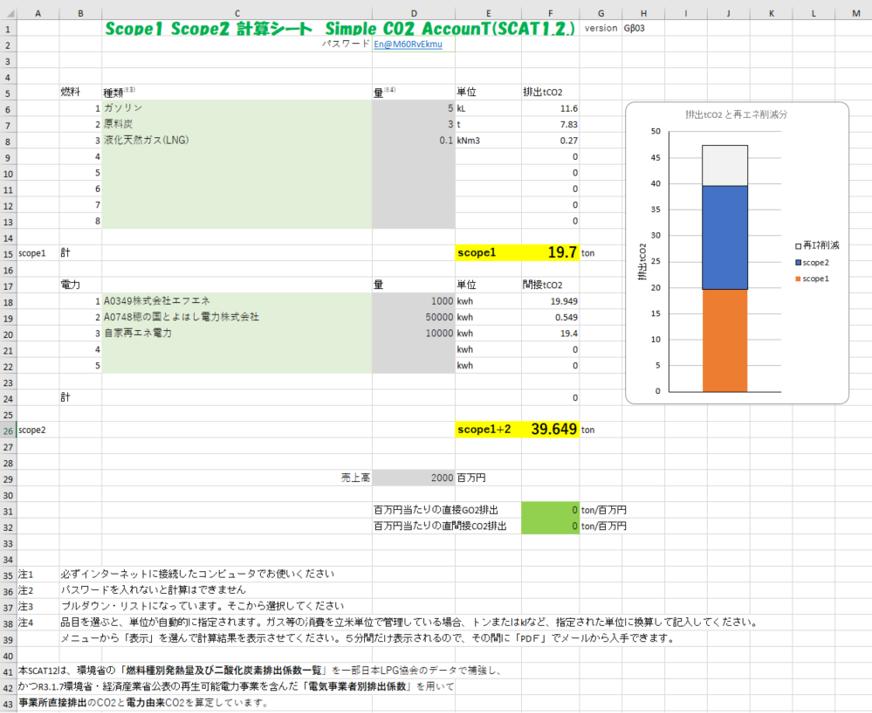
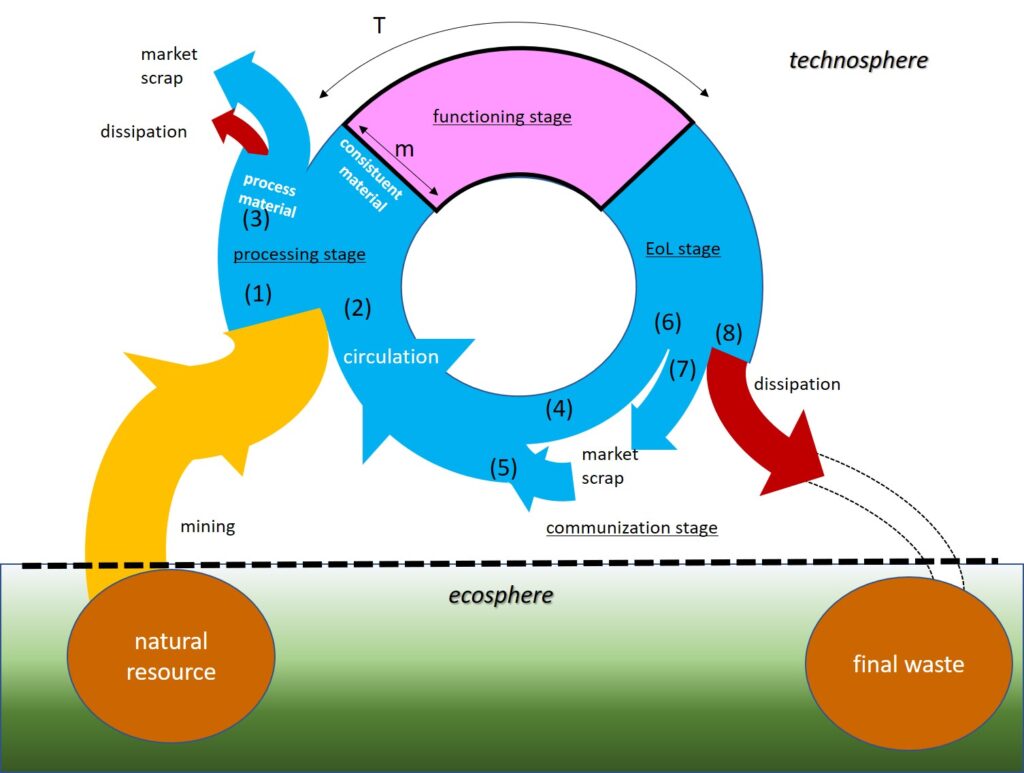
Contents
Organic liquid waste treatment 59
Waste activated carbon treatment 68
Water-based drilling fluid. 75
Synthetic base drilling fluid. 78
Polystyrene-based ion exchange resin. 95
Polystyrene-based ion exchange resin. 96
Detergent for Membrane Separators 121
Concentrated sludge treatment 122
Nitrogen fertilizer (e.g., urea fertilizer). 127
Alkali catalysts (e.g., sodium hydroxide, potassium methylate). 139
Poly aluminum chloride (PAC). 140
Sodium hypochlorite (NaClO). 142
Percentage of composition to the global average of 1 kWh of electricity generated. 146
Coal-fired power generation 1 kWh (considering equipment lifetime). 148
Natural gas-fired power generation (considering equipment lifetime). 150
Oil-fired power generation (taking into account the useful life of equipment). 152
Biomass power generation (taking into account the useful life of equipment). 154
Nuclear power generation (taking into account the useful life of equipment). 156
Photovoltaic and wind power (considering equipment lifetime). 158
Uranium fuel (enriched uranium). 165
Silicon for photovoltaic (PV) power generation. 167
Glass (common soda-lime glass). 169
Composite materials for wind power blades 172
Crushed stone (for construction materials). 175
Admixture (e.g., water reducers and plasticizers for concrete). 176
Hydrogenation catalysts (e.g., palladium catalysts, ruthenium catalysts, etc.). 178
Natural uranium concentrate (yellowcake: U₃O₈). 179
DU (Depleted Uranium) Isolation Processing of Rock Salt Mine Site. 182
High purity quartz (SiO₂) for PV raw materials 187
Carbon electrode for smelting (Graphite electrode) 190
Red mud (bauxite residue) treatment 192
Treatment of waste carbon electrodes (graphite electrodes, etc.). 194
Quartz sand (SiO₂)… 203
Dolomite concentrate (CaMg(CO₃)₂)… 205
Copper concentrate (Cu concentrate)… 206
Glass fiber… 208
Epoxy resin… 210
Hardener (e.g. polyamine, polyamide, etc.).. 211
Carbon fiber for reinforcement (e.g. PAN-based carbon fiber)… 213
Light oil… 214
Polycarboxylic acid ether (PCE)… 216
Preservatives (e.g. paraben, isothiazolinone, formaldehyde) … 218
Palladium chloride (PdCl₂). 219
Gamma-alumina (γ-Al₂O₃). 221
Kerosene (kerosene) .. 222
Hydrofluoric acid (HF) … 224
Potassium Fluoride (KF). 225
Fluorine-containing Residue Treatment.. 227
Quartz Ore Concentrate… 228
Hydrofluoric Acid (HF Acid)… 230
Petroleum Coke… 231
Tar Pitch… 233
Waste Tar Treatment… 235
Polymer Coagulants (e.g. Polyacrylamide)…. 236
Dust Suppressants… 238
Alkali Waste Liquid Treatment.. 239
Flotation Agents, Coagulants, etc. 241
Borax (sodium tetraborate, Na₂B₄O₇·10H₂O)… 242
Binders (e.g. cement, carbon product binders, pellet manufacturing binders)… 244
Bisphenol A (BPA). 245
Epichlorohydrin (Epichlorohydrin, C₃H₅ClO). 247
Ricinoleic Acid (Ricinoleic Acid). 248
Stabilizers (e.g. for polymers, pharmaceuticals, food, etc.)… 250
Stabilizers for polymers.. 252
Polyacrylonitrile (PAN)… 253
Oxidation promoters for organic reactions.. 255
Desulfurization catalysts (typically molybdenum-based catalysts, Co-Mo/Al₂O₃ catalysts, etc.).. 256
Ethylene Glycol (Ethylene Glycol, C₂H₆O₂) 258
Polymerization catalysts (e.g. dialkyl aluminum compounds, Ziegler-Natta catalysts)… 260
Parabens. 261
Acetic acid. 263
Palladium (Pd). 264
Fluorspar (CaF₂). 266
Potassium hydroxide (KOH). 267
Calcium hydroxide (Ca(OH)₂). 269
Heavy fuel oil (HFO)… 270
Catalysts for fluid catalytic cracking (FCC) … 272
Treatment of volatile organic compounds (VOC)… 274
Coal tar… 275
Neutralizers… 277
Surfactants… 278
Polymer Binders.. 280
Polyethylene Glycol (PEG). 281
Mineral Oil… 283
Borate Minerals… 285
Acids and Alkalis… 286
Starch… 288
Ethylene Dichloride (EDC, C₂H₄Cl₂)… 289
Castor Oil… 291
Hindered Phenol… 293
Phosphate Ester… 294
Acrylonitrile (CH₂=CH-CN).. 296
Metal Acrylate (CH₂=CH-CN).. 298
Potassium Persulfate (K₂S₂O₈).. 300
Polyvinyl Alcohol (PVA).. 301
Benzoyl Chloride (C₇H₅ClO) … 303
Calcium Chloride (CaCl₂) .. 305
Molybdenum Trioxide (MoO₃) … 306
Cobalt(II) Nitrate (Co(NO₃)₂) .. 308
Barium oxide (BaO)… 309
p-Hydroxybenzoic acid (p-Hydroxybenzoic acid, PHBA)… 311
Carbon monoxide (CO)… 313
Ruthenium iodide (RuI₃)… 314
Platinum Group Metals (PGM) concentrate… 316
Fluorite (CaF₂) concentrate… 317
Polymer flocculants (e.g. polyacrylamide)… 319
Potassium chloride (KCl)… 321
Silica (SiO₂)… 323
Palladium (Pd) catalyst… 325
Alkylbenzene (Linear Alkylbenzene, LAB)… 327
Vinyl acetate (VAc)… 329
Butadiene (C₄H₆)… 331
Copper chloride (CuCl₂)… 333
Castor seed (Ricinus communis)… 335
Isobutylene (C₄H₈).. 337
Phosphoric acid-based catalysts (1kg) 339
Phosphoric acid (P₂O₅)… 340
Bismuth-molybdenum oxide catalysts (Bismuth-molybdenum oxide catalysts)… 342
Processing of cyanide-containing waste… 344
Zinc oxide (ZnO)… 346
Acid catalysts (e.g. zeolite-based or sulfuric acid-supported catalysts)… 348
Potassium sulfate (K₂SO₄)… 350
Cobalt oxide (Co₃O₄)… 352
Vinyl acetate monomer (VAM)… 354
Benzoic acid… 356
Iron chloride (FeCl₃)… 358
Dichloromethane (CH₂Cl₂)… 360
Chlorine gas treatment… 361
Calcium carbonate (CaCO₃)… 363
Molybdenum concentrate (MoS₂)… 365
Cobalt… 367
Barium carbonate… 368
Titanium tetrachloride (TiCl₄)… 370
Magnesium chloride (MgCl₂)… 372
Triethylaluminum (TEAl)… 373
Copper oxide (CuO)… 375
Nickel catalyst (Ni catalyst)… 377
Ruthenium (Ru)… 379
Iodine (I₂)… 380
Ruthenium chloride (RuCl₃)… 382
Acetonitrile (CH₃CN)… 384
Acrylamide (CH₂=CHCONH₂) 386
Potash ore concentrate… 388
Palladium nitrate.. 389
Alpha olefin… 391
Ethylbenzene… 393
Seeds for sowing… 395
Seeds… 397
Pesticides… 399
MTBE (Methyl Tertiary Butyl Ether)… 401
Phosphate Ore Concentrate… 402
Calcium Oxide (CaO)… 403
Molybdenum Ore Concentrate… 404
Bismuth Ore Concentrate… 405
Zinc Ore Concentrate… 406
Silica ore concentrate… 407
Potassium salt ore concentrate.. 408
Cobalt ore concentrate.. 409
Manganese oxide catalyst… 410
Copper catalyst… 411
Solvent for extracting cobalt… 412
Barium sulfate concentrate… 413
Ilmenite concentrate… 414
Salt lake… 415
Copper concentrate… 416
Nickel concentrate… 418
Ammonium hydroxide (NH₄OH)… 419
Iodine-containing concentrate… 420
Acetamide… 421
Palladium… 423
Aluminum chloride (AlCl₃)… 424
Plant cultivation (1kg)… 425
Phosphate… 426
Fungicide… 428
Manganese ore… 429
Decanoic acid (C₁₀H₂₀O₂)… 430
Octanol (C₈H₁₇OH). 431
Tributyl Phosphate (TBP). 432
Helium gas… 433
Liquid nitrogen… 435
Neon… 435
Argon… 436
Krypton (Kr). 437
Xenon (Xe)… 438
Radon… 439
Bromine (Br₂). 440
Iodine (I₂)… 441
Brine (concentrated salt water)… 443
Sodium Lauryl Sulfate (SLS)… 444
EDTA (Ethylenediaminetetraacetic acid)… 445
Lauric alcohol (dodecanol). 446
Sulfur trioxide (SO₃). 447
Coconut oil (1kg). 448
Dilute sulfuric acid (1kg). 449
V₂O₅ (vanadium pentoxide) catalyst.. 450
Sodium metal (Na)… 451
Coconut Fruit… 453
Vanadium Concentrate.. 454
Lithium Chloride (LiCl). 455
Lithium Concentrate… 457
Selenium… 458
Copper Anode Sludge… 459
Tellurium… 460
Elemental Phosphorus… 461
Industrial Arsenic… 462
Antimony (Sb)… 464
Arsenic Concentrate… 465
Iron Oxide (Fe₂O₃)… 466
Coke… 466
Antimony 1kg… 467
Antimony Concentrate… 469
Bismuth 1kg. 470
Bismuth concentrate… 471
Carbon for industrial use… 472
Silicon for industrial use… 473
Rare earth concentrate… 474
Radioactive material processing… 476
Typical separation procedure for rare earth elements using solvents and pH adjustment… 477
Silica concentrate… 480
Germanium… 482
Tin… 483
Tin ore (SnO₂)… 484
Lead… 485
Lead ore… 487
Boric acid (H₃BO₃)… 488
Borax (boron concentrate)… 489
Aluminum ingot… 491
Aluminum fluoride (AlF₃)… 492
Sodium fluoride (NaF)… 494
Gallium… 495
Indium… 497
D2EHPA (diethylhexyl phosphate)… 500
Methyl isobutyl ketone (MIBK)… 501
2-Ethylhexanol (2-EH)… 502
Copper-chromium catalyst… 504
Butyl aldehyde… 505
Copper nitrate (Cu(NO₃)₂)… 507
Chromium nitrate (Cr(NO₃)₃)… 508
Tetrahydrofuran (THF)… 509
Rhodium catalyst… 511
Chromium concentrate… 513
1,4-Butanediol (BDO)… 514
PGM (Platinum Group Metals) Concentrate.. 516
Acetylene… 517
Xanthate. 519
Calcium Carbide (CaC₂). 520
Carbon Disulfide (CS₂). 522
Thallium (Tl). 523
Zinc… 524
Cadmium… 526
Cinnabar concentrate… 528
Copper… 530
Sulfur dioxide (SO₂) treatment… 531
Calcium hydroxide (Ca(OH)₂). 533
Quicklime (CaO). 534
Silver (Ag). 535
Silver ore (Ag). 536
Gold… 538
Gold ore… 539
Cyanide treatment… 540
Iron(II) sulfate. 542
Cobalt… 544
Cobalt ore… 545
Foam agent for mineral processing… 546
Nickel… 547
Nickel concentrate… 549
Ruthenium… 550
Rhodium… 551
Osmium… 553
Iridium… 554
Platinum… 556
Tributyl phosphate (TBP)… 558
Butanol (1-butanol)… 559
Tributylamine (TBA)… 560
Manganese… 562
Manganese ore… 563
Rhenium… 564
Chromium… 566
Dust treatment… 567
Molybdenum… 568
Tungsten… 569
Tungsten ore… 570
Niobium… 571
Niobium Ore… 572
Tantalum… 573
Tantalum Ore… 574
Titanium… 575
Ilmenite Ore… 576
Magnesium… 577
Magnesium Chloride… 579
Magnesite Ore… 580
Zirconium… 581
Zircon sand… 583
Hafnium… 584
Calcium sulfate (CaSO₄)… 586
Rubidium chloride (RbCl)… 587
Rubidium-bearing concentrate… 589
Cesium chloride… 590
Cesium-bearing concentrate… 591
Beryllium… 593
Beryllium concentrate… 594
Strontium carbonate (SrCO₃). 595
Ceres concentrate (SrSO₄). 596
Salt waste water treatment… 598
Defoaming agent… 599
Polyether Compounds.. 600
Polysorbate… 601
Propylene Oxide.. 603
Sorbitan… 604
Sorbitol… 606
Barium Sulfate (BaSO₄).. 607
Scandium… 608
Bastnaesite Ore… 610
Radium… 612
Uranium ore concentrate… 613
Long-term isolation of radioactive sludge (more than 1000 years)… 615
Glass matrix material for cement solidification… 617
Rare earth ore concentrate… 618
Yttria 1kg… 619
Ceria (CeO₂). 621
Ammonium Oxide (NH₄NO₃).. 623
Lanthanum Oxide (La₂O₃) 624
Neodymium Metal (Nd). 625
Calcium Metal.. 626
Praseodymium Metal.. 627
Samarium Metal.. 629
Gadolinium oxide (Gd₂O₃)… 631
Terbium (Tb). 633
Dysprosium (Dy). 634
Erbium oxide (Er₂O₃). 636
Thorium concentrate… 640
Uranium oxide (U₃O₈)… 641
hydrogen
Currently, there are several ways to produce hydrogen industrially, but the following three are representative.
Steam Methane Reforming (SMR) from natural gas
In this method, natural gas (mainly methane) reacts with water vapor to produce hydrogen.
Reaction equation: CH4+H2O→CO+3H2\text{CH}_4 + \text{H}_2\text{O} \rightarrow \text{CO} + 3\text{H}_2CH4+H2O→CO+3H2
Additional hydrogen is then produced by the reaction of CO (carbon monoxide) and water vapor.
Substance(s) input:.
- Natural Gas (Methane): Approximately 4.5 to 5.0 kg of methane is required.
- Water vapor (H₂O): Requires about 9 kg of water (used as steam).
Type and amount of energy:.
- It is mainly supplied as thermal energy; it takes 7.2-10 kWh of energy to produce 1 kg of hydrogen. Natural gas itself is also used as an energy source, emitting about 9-11 kg of CO₂ as a byproduct.
Solvents and their recirculation rates
- **Water (steam)** is used as a reactant, but the circulation rate is typically 0%. Water used in the reaction is consumed.
- electrolysis of water
This method uses electrolysis to break down water into hydrogen and oxygen. It is also used to produce “green hydrogen” using renewable energy (wind and solar power).
Reaction equation: 2H2O→2H2+O22\text{H}_2\text{O} \rightarrow 2\text{H}_2 + \text{O}_22H2O→2H2+O2
Substance(s) input:.
- Water (H₂O): Approximately 9 kg of water is required.
Type and amount of energy:.
- Electrical energy is required; 50-55 kWh of electricity is needed to produce 1 kg of hydrogen (theoretical value is 39.4 kWh, but it takes more energy considering the actual process efficiency).
Solvents and their recirculation rates
- Water is used as a reactant, but is consumed, resulting in a circulation rate of 0%.
- biomass gasification
This process produces hydrogen by treating biomass at high temperatures. By vaporizing the biomass, synthesis gas containing hydrogen, carbon monoxide, and carbon dioxide is produced, and hydrogen is obtained in a subsequent reaction.
Reaction formula: C6H10O5+H2O→H2+CO+CH4\text{C}_6\text{H}_{10}\text{O}_5 + \text{H}_2\text{O} \rightarrow \text{H}_2 + \text{CO} + \text{CH}_4C6 H10O5+H2O→H2+CO+CH4
Substance(s) input:.
- Biomass (wood, agricultural waste, etc.): approx. 6-12 kg
- Water vapor (H₂O): Requires about 9 kg of water.
Type and amount of energy:.
- Thermal energy is the main source of energy used; approximately 10-13 kWh of energy is required to produce 1 kg of hydrogen.
Solvents and their recirculation rates
- Water is used as the main solvent, but the circulation rate is low (0%) because it is consumed in the reaction.
oxygen
The most common method for industrial production of oxygen is the Air Separation Process. There are two main types of air separation processes: cryogenic air separation and newer methods such as membrane separation and adsorption separation. The most common Cryogenic Air Separation Process is described in detail below.
Cryogenic Air Separation
In this method, air is cooled, liquefied, and separated into its components, including oxygen, nitrogen, and argon.
Process Overview
- Air compression: draws in air from outside and compresses it.
- Air cooling: compressed air is cooled to liquid air.
- Distillation: Liquid air is distilled to separate oxygen, nitrogen, and argon. Oxygen is separated faster than other components because of its relatively high boiling point.
Substance(s) to be input
- Atmospheric Air: Air is taken directly from the atmosphere to produce oxygen. The oxygen content in air is about 21%; it takes about 4.76 kg of air to produce 1 kg of oxygen (based on 21% oxygen content).
Type and amount of energy
- Electrical energy is the primary requirement. To produce 1 kg of oxygen, electricity for compression, cooling, and distillation is consumed in the range of 0.4 to 1.0 kWh/kg. Although it varies depending on the efficiency of the process, energy in the range of 0.6 to 0.9 kWh/kg is typical for standard industrial processes.
Solvents and their circulation rates
- **Refrigerant (e.g., liquid nitrogen)** may be used as a cooling medium to liquefy air, but is circulated. This refrigerant is typically reused in the internal cooling cycle, so the circulation rate is almost 100%.
- pressure swing adsorption (PSA)
This process uses an adsorbent to selectively adsorb nitrogen from the air, leaving oxygen behind; PSA does not produce oxygen as pure as the cryogenic air separation method, but it is often used in some applications because it produces oxygen with relatively little energy.
Substance(s) to be input
- Atmospheric air: Approximately 4.76 kg of air is required to produce 1 kg of oxygen.
Type and amount of energy
- Electrical energy is required, and approximately 0.3 to 0.6 kWh of energy is consumed to produce 1 kg of oxygen. Compared to the low-temperature air separation method, less energy is required to obtain oxygen, but the purity is somewhat lower (approximately 90-95%).
Solvents and their circulation rates
- No solvents are used in this process.
- membrane separation method
Membrane separation is a process that uses selective gas permeation to separate oxygen from air. It has the advantage of low energy operation, but is not as popular as the cryogenic air separation method for large-scale industrial applications.
Substance(s) to be input
- Atmospheric air: Approximately 4.76 kg of air is required to separate oxygen.
Type and amount of energy
- As electrical energy, 0.2-0.4 kWh/kg of energy is required to produce 1 kg of oxygen. Although power consumption varies depending on the efficiency of the process, it consumes less energy than the low-temperature air separation method.
Solvents and their circulation rates
- No solvent is used and there is no circulating material because gases are selectively separated through the membrane.
summary
| method | Input Substances | Energy consumption (kWh/kg) | Solvent type and circulation rate |
| Cryogenic Air Separation Method | Air approx. 4.76 kg | 0.6 to 0.9 | Refrigerant such as liquid nitrogen (100% circulation) |
| Adsorption separation method (PSA) | Air approx. 4.76 kg | 0.3 to 0.6 | Solvent free |
| Membrane separation method | Air approx. 4.76 kg | 0.2 to 0.4 | Solvent free |
The low-temperature air separation method is the standard method that can provide high-purity oxygen, but it has high energy costs. On the other hand, adsorption and membrane separation methods are more energy efficient, but tend to provide slightly less pure oxygen.
lithium carbonate (Li2CO3)
The general process for producing lithium carbonate (Li₂CO₃) is primarily by extraction from lithium ores or from lithium salt lakes. A typical process for production using the evaporation and concentration process from lithium salt lakes is shown below.
- process for production of Li₂CO₃ from lithium salt lakes
In this method, salt water from a salt lake (primarily brine containing lithium) is evaporated to concentrate the lithium to obtain Li₂CO₃.
Substance(s) to be input
- Salt water (lithium content 0.1% to 0.2%): Approximately 7 to 15 tons of salt water is required (depending on lithium concentration).
- In addition to lithium, lithium-containing brine contains impurities such as sodium, potassium, magnesium, and calcium, which must be removed.
- Sodium carbonate (Na₂CO₃): needed to precipitate lithium in the form of Li₂CO₃. Approximately 0.75 kg of sodium carbonate is used to produce 1 kg of Li₂CO₃.
Type and amount of energy
- Solar energy: The evaporation pond evaporates the water in the salt water and concentrates the lithium. The evaporation itself uses solar energy, so little external energy is required.
- Electrical energy: required for concentration, pumping, filtering, and precipitation with sodium carbonate. Electricity consumption depends on the efficiency and scale of the process, but the electricity required to produce 1 kg of Li₂CO₃ is 6-8 kWh.
Process substances and their circulation rates
- Brine: The input brine is not circulated because lithium is concentrated by evaporation. Residual salt is treated separately.
- Sodium carbonate (Na₂CO₃): Consumed in the precipitation reaction, so it is not used in circulation.
- Water: may be used for cleaning and filtering in some processes, but mostly consumed by evaporation.
- process for producing Li₂CO₃ from lithium ore (spiliomite)
Another process involves treating lithium ore (spiliomite, LiAlSiO₄) at high temperatures to dissolve and purify the lithium to obtain Li₂CO₃.
Substance(s) to be input
- Spiriomite ore (Li₂O content: approx. 1.5%): approx. 7 kg of spiriomite ore is required to produce 1 kg of Li₂CO₃.
- Sulfuric Acid (H₂SO₄): Used to dissolve lithium ore and extract lithium. Approximately 0.8 to 1.0 kg of sulfuric acid is used to produce 1 kg of Li₂CO₃.
Type and amount of energy
- Thermal energy: required to sinter the ore at high temperatures and chemically process the lithium; the thermal energy required to produce 1 kg of Li₂CO₃ is about 8-10 kWh.
- Electrical energy: Electricity required for machine operations such as grinding, concentration, filtering, pumping, washing, etc. Approximately 5-7 kWh are required.
Process substances and their circulation rates
- Sulfuric acid (H₂SO₄): Sulfuric acid is consumed by the reaction, so the circulation rate is 0%.
- Water: In the refining process, wash water is used, but is rarely circulated because it is consumed by reactions.
- Lime (CaO): used for impurity removal in some processes, but consumed
summary
| method | Input Substances | Energy consumption (kWh/kg) | Solvents and their circulation rates |
| Production from lithium salt lakes | Salt water: approx. 7-15 tons Sodium carbonate: approx. 0.75 kg |
Electric energy: 6-8 kWh | Water evaporates and circulation rate 0% Na₂CO₃ is consumed |
| Production from lithium ore | Spiriomite ore: approx. 7 kg Sulfuric acid : approx. 0.8-1.0 kg |
Thermal energy: 8-10 kWh Electrical energy: 5-7 kWh |
Sulfuric acid is consumed Water is consumed |
Thus, the extraction method from lithium salt lakes is relatively energy efficient and uses natural energy (sunlight), thus consuming less external energy. On the other hand, the extraction method from ores requires high-temperature processing and thus consumes more energy.
Below is a quantitative estimate of the materials input and consumed in the production of 1 kg of lithium carbonate (Li₂CO₃).
- process for production of Li₂CO₃ from lithium salt lakes
Substance(s) to be input and its quantity
- Salt water:
- 7-15 tons of salt water is required (depending on lithium concentration of 0.1%-0.2%).
- This brine is eventually consumed as the lithium is concentrated by evaporation.
- Sodium carbonate (Na₂CO₃):
- 0.7-0.8 kg of sodium carbonate is fed to produce 1 kg of Li₂CO₃, which is consumed when lithium ions are precipitated into lithium carbonate.
Energy consumption
- Electrical energy:
- 6-8 kWh/kg of electricity is consumed. This is required primarily for the operation of pumps, filters, and concentrators.
- Solar energy:
- No energy is consumed externally for the natural evaporation of water in the evaporation pond (using natural solar energy).
Process substances and circulation rates
- Water:
- The salt water in the salt lake is concentrated by evaporation and eventually Li₂CO₃ is extracted. Water is consumed and the circulation rate is 0%.
- Sodium carbonate (Na₂CO₃):
- It is consumed in the precipitation reaction and is not reused (0% recirculation rate).
- process for producing Li₂CO₃ from lithium ore (spiliomite)
Substance(s) to be input and its quantity
- Spiriomite ore (1.5% Li₂O content):
- 7-8 kg of spiliomite ore is required. The ore is completely consumed as it is calcined and dissolved in sulfuric acid.
- Sulfuric acid (H₂SO₄):
- 0.8 to 1.0 kg of sulfuric acid is required to dissolve the lithium. Sulfuric acid is consumed and not circulated in the process.
Energy consumption
- Thermal energy:
- 8-10 kWh/kg of thermal energy is required for calcination of ore and concentration of lithium.
- Electrical energy:
- It consumes 5 to 7 kWh/kg of electricity. This is used for grinding, filtering, pumping material, and refining.
Process substances and circulation rates
- Sulfuric acid (H₂SO₄):
- It is used to dissolve lithium ore and is not circulated as it is consumed (0% circulation).
- Water:
- It is used and consumed in the cleaning and cooling processes. The circulation rate is 0%.
- Lime (CaO):
- Used for impurity removal in some processes, but consumed (0% circulation).
summary
| Manufacturing Method | Input Substances | Input (kg/1kg Li₂CO₃) | Substance and amount consumed | Energy consumption (kWh/kg) | Solvents and circulation rates |
| Production from lithium salt lakes | Salt water: approx. 7-15 tons | Salt water: approx. 7-15 tons | All evaporated and consumed. | Electric energy: 6-8 kWh | Water is consumed and circulation rate is 0%. |
| Sodium carbonate: approx. 0.7-0.8 kg | Sodium carbonate: approx. 0.7-0.8 kg | The entire amount is consumed in the precipitation reaction. | Solar energy: no external consumption | Na₂CO₃ is consumed and the circulation rate is 0%. | |
| Production from lithium ore | Spiriomite ore: approx. 7-8 kg | Spiriomite ore: approx. 7-8 kg | The entire amount is consumed in dissolution. | Thermal energy: 8-10 kWh | Sulfuric acid is consumed and circulation rate is 0%. |
| Sulfuric acid: approx. 0.8 to 1.0 kg | Sulfuric acid: approx. 0.8 to 1.0 kg | The entire amount is consumed in the dissolution reaction | Electric energy: 5 to 7 kWh | Water is also consumed, circulation rate 0%. |
This table summarizes the amount of input materials, consumed materials, energy consumption, and their recycling rates required to produce Li₂CO₃.
Water consumption in the production of lithium carbonate (Li₂CO₃) from lithium ore (spiliomite) varies with each stage of the process, but can be estimated as a general guide as follows
Water consumption (per kg of Li₂CO₃)
- One to two tons of water is required.
Breakdown:
- Ore dissolution and reaction processes:
- A certain amount of water is used to dissolve the ore with sulfuric acid.
- Cleaning and filtration process:
- Much wash water is consumed to remove ore residues and impurities.
- Water for cooling and process use:
- Water is used for thermal management of reactions and cooling of equipment, which may be partially reused, but is consumed in parts of the process.
The exact amount of water consumed will vary depending on the efficiency of the equipment and process, but as a rule of thumb, approximately 1-2 tons of water will be consumed to produce 1 kg of Li₂CO₃.
The most universal compound of beryllium is **beryllium oxide (BeO)**. Beryllium oxide is widely used industrially because of its excellent heat resistance and electrical insulation properties. The common method for producing this compound is the process of extracting beryllium from beryllium ore (mainly beryl, Be₃Al₂(SiO₃)₆) and converting it to oxide.
- beryllium oxide (BeO) production process
Beryllium-bearing ores such as beryl (Be₃Al₂(SiO₃)₆) are chemically treated to produce beryllium oxide (BeO)
Substance(s) to be input and its quantity
- Beryl ore (Be₃Al₂(SiO₃)₆):
- The beryllium content in beryl is about 3-5%, and 5-10 kg of beryl ore is needed to produce 1 kg of BeO
- Sulfuric acid (H₂SO₄) or hydrofluoric acid (HF):
- It is used to dissolve beryllium and remove impurities such as aluminum and silica.
- Sulfuric acid and hydrofluoric acid are typically used in quantities of approximately 2 to 4 kg and 0.5 to 1.0 kg, respectively.
- Ammonia (NH₃):
- It is used to neutralize the reaction and precipitate the beryllium. Approximately 0.5 to 1.0 kg of ammonia is used.
Type and amount of energy
- Thermal energy:
- It is used for calcination and melting processes of ores. Calcination temperatures of approximately 900-1200°C require thermal energy of 10-15 kWh/kg.
- Electrical energy:
- It is used in processes such as grinding, dissolution, filtration, precipitation, filter drying, etc. To produce 1 kg of BeO consumes 8-12 kWh/kg of electricity.
Process substances and their circulation rates
- Water:
- Water is used in the cleaning and dissolution process; approximately 1-2 tons of water is required to produce 1 kg of BeO Although it may be reused in some parts of the process, much is ultimately consumed (the recirculation rate is low, roughly 0%).
- Sulfuric acid (H₂SO₄) or hydrofluoric acid (HF):
- These acids are consumed and not reused (0% recirculation).
- Ammonia (NH₃):
- It is consumed in the precipitation reaction of beryllium, but a portion may be recovered and reused through post-chemical processing. The recycling rate is estimated to be **about 30-50%**.
summary
| Input Substances | Amount (kg/1 kg BeO) | Energy consumption (kWh/kg) | Solvents and their circulation rates |
| beryl ore | 5-10 kg | – | Consumed and circulation rate 0%. |
| Sulfuric acid (H₂SO₄) or hydrofluoric acid (HF) | Sulfuric acid: 2-4 kg Hydrofluoric acid: 0.5-1.0 kg |
– | Consumed and circulation rate 0%. |
| Ammonia (NH₃) | 0.5 to 1.0 kg | – | Reuse rate: approx. 30-50 |
| water (esp. cool, fresh water, e.g. drinking water) | 1 to 2 tons | – | Consumed and circulation rate 0%. |
| thermal energy | – | 10-15 kWh/kg | – |
| electric energy | – | 8-12 kWh/kg | – |
This table summarizes the approximate amount of input materials and energy consumed, as well as the solvent that can be recycled, to produce 1 kg of beryllium oxide (BeO). The values may vary up or down depending on the efficiency of the process and equipment.
methane
Below are approximate figures for the industrial production of 1 kg of methane (CH₄). The method described here is based primarily on the Methanation Process (Sabatier reaction)**, which synthesizes methane from **hydrogen and carbon dioxide. This process is used in a variety of industrial applications, including the production of methane from synthesis gas using renewable energy sources.
Methane production by methanation process
Reaction equation:
CO2+4H2→CH4+2H2O\text{CO}_2 + 4\text{H}_2 \rightarrow \text{CH}_4 + 2\text{H}_2\text{O}CO2+4H2→CH4 +2H2O
input
| Name of input | Suggested input amount |
| Carbon dioxide (CO₂) | 2.75 kg (theoretical value: 44 g/mol) |
| Hydrogen (H₂) | 0.5 kg (theoretical value: 4 mol = 8 g/mol) |
Solvents and other process substances
| name | Amount used (kg) | Cycloparametric rate |
| Catalysts (Ni-based catalysts, Ru-based catalysts, etc.) | No catalyst is consumed, so no additional volume is added. Typically replenishes several tens of grams to several kilograms/reactor per year | Almost 100% (can be used for a long period of time) |
| Water (for cooling and heating) | Approx. 10-15 kg | 70-90% of the total |
Type and amount of energy
| Energy Type | amount of consumption |
| Electricity (kWh/kg) | Approx. 4-6 kWh (mainly used for temperature control of hydrogen compression and methanation processes) |
Fuel type and quantity
| type | Quantity (kg) |
| No fuel is used (mainly for electricity consumption, no fuel for combustion) | – |
Other than combustion, CO₂
| Amount generated (kg) |
| Zero CO₂ produced during the reaction (all converted to methane and water) |
Waste requiring treatment
| name | Approximate quantity |
| Waste catalyst (at end of process) | Approx. 10-20 g/year (depending on catalyst life at industrial scale) |
| Impurity removal sludge (pretreatment of CO₂ and H₂) | Approx. 10-50 g/kg (depending on impurity content) |
Learn more about the metanation process
Theoretical quantities of carbon dioxide and hydrogen are required to produce 1 kg of methane. In addition, electricity consumption is used primarily for hydrogen compression and temperature control of the reaction. To maximize process efficiency, Ni-based catalysts are often used, and since these are long-lasting, small amounts of waste catalyst are generated during periodic catalyst changes.
remarks
- Energy consumption for hydrogen production (e.g., electrolysis) is not included.
- Since water for cooling and heating is usually used in a circulation system, most of the input is reused, resulting in lower final consumption.
Methane production from natural gas generally refers to the process of refining or reforming natural gas to increase its purity and supply it in a usable form. Since natural gas is primarily composed of methane (CH₄), the process is more akin to purification, separation, and concentration than production.
Below are the inputs and energy consumption for obtaining 1 kg of pure methane through the natural gas refining process, organized by each element.
Methane production by natural gas refining process
input
| Input Item Name | Suggested input amount |
| Natural gas (feedstock gas) | 1.1-1.3 kg (70-95% purity of methane content) |
| Air or oxygen (for oxidation and hydrogen sulfide removal) | 0.05 to 0.2 kg (for hydrogen sulfide oxidation) |
Solvents and other process substances
| name | Approximate amount used (kg) | Cycloparametric rate |
| Water (for gas cleaning, cooling, heating) | 3-5 kg | 50-70% (e.g., cooling water) |
| Amine solution (for CO₂ removal) | 0.5 to 1.0 kg | 90-95% of |
| Dimethyl ether (DME) or MEG | 0.3 to 0.5 kg | 90-95% (often reused) |
Type and amount of energy
| Energy Type | Approximate consumption |
| Electricity (kWh/kg) | 2-4 kWh (mainly used for pumps, compressors, and gas cooling) |
| Thermal energy (e.g., natural gas combustion) | 1 to 2 kWh (used for hydrogen sulfide removal, heating, etc.) |
Fuel type and quantity
| type | Quantity (kg) |
| Natural gas (for own consumption) | 0.05-0.1 kg/kg methane |
Other than combustion, CO₂
| Amount generated (kg) |
| 0.2-0.3 kg (as a byproduct during CO₂ separation and refining) |
Waste requiring treatment
| name | Approximate quantity |
| Sulfur sludge (after H₂S removal) | 0.01-0.02 kg/kg Methane |
| Sludge containing water and impurities | 0.01-0.05 kg/kg Methane |
Process Details
- Gas cleaning:
Since natural gas often contains impurities such as hydrogen sulfide (H₂S), these impurities are removed by amine treatment or oxidation. The amine solutions used (e.g., MEA, DEA) are typically reused in the process, with a circulation rate of over 90%. - Gas separation and purification:
Membrane separation, cryogenic separation (LNG process), etc. are used to remove components other than methane (CO₂, H₂S, nitrogen, helium, etc.). These processes consume electricity and thermal energy for cooling. - Gas Cooling and Heating:
Cooling water and heat transfer fluids are used in the cooling and heat exchange processes. Cooling water has a high recirculation rate and the heat transfer fluid for heating (e.g. DME, MEG) is often reused. - Final Purification:
After purification, the methane is recovered and shipped in its final, high-purity form.
summary
| Input Item Name | Suggested input amount |
| Natural gas (feedstock gas) | 1.1 to 1.3 kg |
| Air or oxygen | 0.05 to 0.2 kg |
| Solvents and other process substances Name | Approximate amount to be used | Cycloparametric rate |
| Water (for gas cleaning and cooling) | 3-5 kg | 50-70% (cooling system) |
| Amine solution (for CO₂ removal) | 0.5 to 1.0 kg | 90-95% of |
| Dimethyl ether (DME) or MEG | 0.3 to 0.5 kg | 90-95% of |
| Type and amount of energy | Approximate consumption |
| Electricity (kWh/kg) | 2 to 4 kWh |
| Thermal energy (natural gas combustion) | 1 to 2 kWh |
| Fuel Type | Approximate quantity |
| Natural gas (for own consumption) | 0.05 to 0.1 kg |
| 0.2 to 0.3 kg | CO₂ other than combustion
| Waste requiring treatment Name | Approximate quantity |
| sulfur sludge | 0.01 to 0.02 kg |
| Sludge containing water and impurities | 0.01 to 0.05 kg |
The following is a list of the most common problems with the
water vapor
Industrial steam production typically involves the use of boilers to heat water and convert it into steam. The process uses water and fuel (gas, coal, biomass, etc.) as inputs and consumes thermal energy and electricity.
The following is an organized list of approximate values for the industrial production of 1 kg of steam.
Industrial steam production process (boiler)
input
| Input Item Name | Suggested input amount |
| Water (H₂O) | 1.05 to 1.15 kg (including loss due to evaporation) |
Solvents and other process substances
| name | Approximate amount used (kg) | Cycloparametric rate |
| Boiler chemicals (rust inhibitors, oxygen absorbers, etc.) | 0.01 to 0.03 kg | Almost 0% (consumed) |
| Water (for cooling) | 5-10 kg | 90-95% (circulation use) |
Type and amount of energy
| Energy Type | Approximate consumption |
| Electricity (kWh/kg) | 0.01 to 0.05 kWh/kg (for pumps and controls) |
| Thermal energy (fuel-derived) | 2.2 to 2.7 kWh/kg (theoretical heat value 2.26 kWh/kg, based on specific heat of water) |
Fuel type and quantity
| type | Approximate quantity (kg) |
| Natural gas (at 85% thermal efficiency) | 0.08 to 0.1 kg |
| Heavy oil (at 80% thermal efficiency) | 0.1 to 0.13 kg |
| Coal (at 75% thermal efficiency) | 0.11 to 0.15 kg |
Other than combustion, CO₂
| Amount generated (kg) |
| Zero (no CO₂ is generated except from fuel combustion) |
Waste requiring treatment
| name | Approximate quantity |
| Sludge (scale component) at the bottom of boiler | 0.01 to 0.02 kg/kg Water vapor |
| Chemical waste (after chemical cleaning) | 0.005 to 0.01 kg/kg Water vapor |
summary
| Input Item Name | Suggested input amount |
| Water (H₂O) | 1.05 to 1.15 kg |
| Solvents and other process substances Name | Approximate amount to be used | Cycloparametric rate |
| Boiler chemicals (rust inhibitors, oxygen absorbers, etc.) | 0.01 to 0.03 kg | Almost 0 |
| Water (for cooling) | 5-10 kg | 90-95% (reused) |
| Type and amount of energy | Approximate consumption |
| Electricity (kWh/kg) | 0.01 to 0.05 kWh |
| Thermal energy (fuel-derived) | 2.2 to 2.7 kWh |
| Fuel Type | Approximate quantity (kg) |
| Natural gas (85% thermal efficiency) | 0.08 to 0.1 kg |
| Heavy oil (80% thermal efficiency) | 0.1 to 0.13 kg |
| Coal (75% thermal efficiency) | 0.11 to 0.15 kg |
| 0 kg | CO₂ other than combustion
| Waste requiring treatment Name | Approximate quantity |
| Sludge (scale component) at the bottom of boiler | 0.01 to 0.02 kg |
| Chemical waste (after chemical cleaning) | 0.005 to 0.01 kg |
natural gas
The process typically used to industrially produce natural gas (the main component is methane) is the “natural gas extraction and purification process. In this process, natural gas is extracted from underground gas fields and impurities (hydrogen sulfide, carbon dioxide, moisture, etc.) are removed at a processing facility to produce natural gas as a product.
Below are approximate figures for producing 1 kg of natural gas by a method commonly used in industry.
Natural gas extraction and refining processes
input
| Input Item Name | Suggested input amount |
| Crude oil-bearing gas or gas field gas | 1.2-1.5 kg (80-85% methane purity in gas) |
Solvents and other process substances
| name | Approximate amount used (kg) | Cycloparametric rate |
| Amine solution (for CO₂ removal) | 0.05 to 0.1 kg | 90-95% of |
| Glycol (for dehydration) | 0.05 to 0.1 kg | 90-95% (recycled after reprocessing) |
| Methanol (hydrate prevention) | 0.01 to 0.05 kg | 80-90% (recoverable) |
| Water (for cooling and cleaning) | 2 to 5 kg | 70-90% of the total |
Type and amount of energy
| Energy Type | Approximate consumption (kWh/kg) |
| Electric power (for pumps and compressors) | 0.3 to 0.5 kWh |
| Thermal energy (for gas processing) | 1.0 to 1.5 kWh |
Fuel type and quantity
| type | Approximate quantity (kg) |
| Natural gas (for private consumption) | 0.02 to 0.05 kg |
Other than combustion, CO₂
| Amount generated (kg) |
| 0.1 to 0.3 kg (during CO₂ separation and impurity treatment) |
Waste requiring treatment
| name | Approximate quantity |
| Sulfur sludge (after H₂S removal) | 0.005~0.02 kg/kg Gas |
| Sludge containing water and impurities | 0.01 to 0.05 kg/kg Gas |
| Amine effluent (at obsolescence) | 0.001~0.005 kg/kg Gas |
Process details and guidelines
- Gas Extraction
When extracting gas from underground gas fields or crude oil companion gas, gas is extracted under high pressure and at low temperatures. In addition to methane, the raw gas contains components such as ethane, propane, butane, carbon dioxide, and hydrogen sulfide, which must be separated and purified. - Impurity removal (desulfurization and CO₂ removal)
Amine solutions are used to remove hydrogen sulfide (H₂S) and carbon dioxide (CO₂). The amine solution is circulated through the process and has a circulation rate of 90-95%. - Dehydration Treatment
Glycol is used to remove water from the natural gas. This prevents hydrate formation in natural gas and ensures stability during pipeline transportation. After recovery, the glycol is recycled and treated for reuse (90-95% recirculation rate). - Hydrate prevention (methanol injection)
Methanol is added to prevent water and methane from combining at low temperatures to form hydrates. Some of the methanol used can be recovered, with a circulation rate of 80-90%. - Refining and Compression
The final stage involves compressing the refined natural gas and preparing it for shipment as a product. Electricity and thermal energy are used in this process.
summary
| Input Item Name | Suggested input amount |
| Crude oil-bearing gas or gas field gas | 1.2 to 1.5 kg |
| Solvents and other process substances Name | Approximate amount used (kg) | Cycloparametric rate |
| Amine solution (for CO₂ removal) | 0.05 to 0.1 kg | 90-95% of |
| Glycol (for dehydration) | 0.05 to 0.1 kg | 90-95% of |
| Methanol (hydrate prevention) | 0.01 to 0.05 kg | 80-90% of the total |
| Water (for cooling and cleaning) | 2 to 5 kg | 70-90% of the total |
| Type and amount of energy | Approximate consumption (kWh/kg) |
| electric power | 0.3 to 0.5 kWh |
| thermal energy | 1.0 to 1.5 kWh |
| Fuel Type | Approximate quantity (kg) |
| Natural gas (for private consumption) | 0.02 to 0.05 kg |
| 0.1 to 0.3 kg | CO₂ other than combustion
| Waste requiring treatment Name | Approximate quantity |
| Sulfur sludge (after H₂S removal) | 0.005 to 0.02 kg |
| Sludge containing water and impurities | 0.01 to 0.05 kg |
| Amine effluent (at obsolescence) | 0.001 to 0.005 kg |
amine solution
Amine solutions, such as **monoethanolamine (MEA), diethanolamine (DEA), and triethanolamine (TEA)**, are commonly used in industry to remove carbon dioxide (CO₂) and hydrogen sulfide (H₂S).
Here is a rough estimate of input and energy consumption based on a process for producing 1 kg of **monoethanolamine (MEA)**, a typical amine. Monoethanolamine is produced by reacting **ethylene oxide (EO) with ammonia (NH₃)**.
Monoethanolamine (MEA) Production Process
input
| Input Item Name | Suggested input amount |
| Ethylene oxide (EO) | 0.55 to 0.65 kg |
| Ammonia (NH₃) | 0.25 to 0.35 kg |
| Water (H₂O) | 0.1 to 0.15 kg (for reaction adjustment) |
Solvents and other process substances
| name | Approximate amount used (kg) | Cycloparametric rate |
| Catalyst (acidic or basic catalyst) | 0.01 to 0.05 kg | Almost 100% (reused) |
| Water (for cooling) | 5-10 kg | 90-95% of |
| Solvent for cleaning (e.g., methanol) | 0.05 to 0.1 kg | 70-80% of the total |
Type and amount of energy
| Energy Type | Approximate consumption (kWh/kg MEA) |
| electric power | 0.5 to 1.0 kWh |
| Thermal energy (steam) | 2.5 to 3.0 kWh |
Fuel type and quantity
| type | Approximate quantity (kg) |
| Natural gas or heavy oil (heat source) | 0.1 to 0.15 kg |
Other than combustion, CO₂
| Amount generated (kg) |
| 0 kg (no CO₂ generation during main reaction) |
Waste requiring treatment
| name | Approximate quantity |
| Reaction byproducts (e.g. diethanolamine) | 0.02 to 0.05 kg/kg MEA |
| Catalyst waste (after long-term use) | 0.001 to 0.002 kg/kg MEA |
| Cleaning waste liquids (e.g., methanol waste) | 0.01 to 0.05 kg/kg MEA |
Process details and guidelines
- Main reaction (reaction of ethylene oxide and ammonia)
- Ethylene oxide (EO) reacts with ammonia (NH₃) to produce monoethanolamine (MEA). A small amount of water is used to adjust the reaction.
- Small amounts of diethanolamine (DEA) and triethanolamine (TEA) may be produced as byproducts.
- Cooling and separation process
- The product is cooled and the MEA is purified in a separation process. Cooling water is used in a recirculation system, which has a high recirculation rate (90-95%), although the amount of water used is high.
- Cleaning and solvent use
- Small amounts of cleaning solvents (e.g., methanol) are used to clean the products and reactors after the reaction. These are often reused after collection, but the recirculation rate is about 70-80%.
- Thermal energy consumption
- Steam consumption, mainly used for heating during the reaction and in the distillation and separation processes, is significant, with overall process thermal energy consumption ranging from about 2.5 to 3.0 kWh/kg.
- Byproduct treatment
- Byproducts produced during the reaction, such as diethanolamine (DEA), may be reused in other chemical processes if necessary, but most are separated and treated as waste.
summary
| Input Item Name | Input amount guideline |
| Ethylene oxide (EO) | 0.55 to 0.65 kg |
| Ammonia (NH₃) | 0.25 to 0.35 kg |
| Water (H₂O) | 0.1 to 0.15 kg |
| Solvents and other process substances Name | Approximate amount to be used | Cycloparametric rate |
| Catalyst (acidic or basic catalyst) | 0.01 to 0.05 kg | Almost 100%. |
| Water (for cooling) | 5-10 kg | 90-95% of |
| Solvent for cleaning (e.g., methanol) | 0.05 to 0.1 kg | 70-80% of the total |
| Type and amount of energy | Approximate consumption (kWh/kg) |
| electric power | 0.5 to 1.0 kWh |
| Thermal energy (steam) | 2.5 to 3.0 kWh |
| Fuel Type | Approximate quantity (kg) |
| Natural gas or heavy oil (heat source) | 0.1 to 0.15 kg |
| 0 kg | CO₂ other than combustion
| Waste requiring treatment Name | Quantity standard |
| Reaction byproducts (e.g. DEA) | 0.02 to 0.05 kg |
| Catalyst waste (after long-term use) | 0.001 to 0.002 kg |
| Cleaning waste liquids (e.g., methanol waste) | 0.01 to 0.05 kg |
dimeter ether
Dimethyl ether (DME) is an organic compound produced mainly by the dehydration reaction of methanol, and is widely used in applications such as fuels and refrigerants. Here, we present a summary of the inputs and energy consumption for producing 1 kg of dimethyl ether based on the “DME production process from methanol,” which is commonly used in industry.
DME production process from methanol
Reaction Formula:
2CH3OH→CH3OCH3+H2O2\text{CH}_3\text{OH} \rightarrow \text{CH}_3\text{OCH}_3 + \text{H}_2\text{O}2CH3OH→CH3 OCH3+H2O
input
| Input Item Name | Suggested input amount |
| Methanol (CH₃OH) | 1.9 to 2.1 kg |
| Water (for reaction adjustment) | 0.05 to 0.1 kg |
Solvents and other process substances
| name | Approximate amount used (kg) | Cycloparametric rate |
| Catalyst (acidic catalyst, e.g. γ-Al₂O₃) | 0.01 to 0.02 kg | Almost 100% (can be used for a long period of time) |
| Water (for cooling and heating) | 3-5 kg | 80-95% (circulation used) |
Type and amount of energy
| Energy Type | Approximate consumption (kWh/kg DME) |
| electric power | 0.2 to 0.4 kWh |
| Thermal energy (steam or natural gas) | 1.5 to 2.0 kWh |
Fuel type and quantity
| type | Approximate quantity (kg) |
| Natural gas or fuel oil (heat source) | 0.05 to 0.1 kg |
Other than combustion, CO₂
| Amount generated (kg) |
| 0 kg (no CO₂ is generated except by combustion) |
Waste requiring treatment
| name | Approximate quantity |
| Catalyst waste (after long-term use) | 0.001~0.005 kg/kg DME |
| Process water (byproduct moisture) | 0.1-0.2 kg/kg DME |
Process details and guidelines
- Main reaction (dehydration of methanol)
- Methanol (CH₃OH) is heated in the presence of a catalyst to carry out a dehydration reaction. This reaction produces dimethyl ether (DME) and water (H₂O). The theoretical yield of the reaction is one molecule of DME and one molecule of water from two molecules of methanol, but in the actual process a small amount of unreacted methanol remains.
- Cooling and separation process
- The product is cooled to separate the dimethyl ether from the byproduct, water. Cooling water is used in a recirculation system, so the recirculation rate is high (80-95%), although the amount used is high.
- Recirculation of unreacted methanol
- Unreacted methanol separated after the reaction is often returned to the reaction system again and is rarely discharged as waste.
- Catalyst management
- Acid catalysts (e.g., γ-alumina) are used in DME production and can be used for long periods of time, but after prolonged use the catalyst deteriorates and generates a small amount of catalyst waste.
- Byproduct (water) treatment
- The water produced in the dehydration reaction is either reused as process water or treated in a wastewater treatment facility. This produces a small amount of wastewater.
summary
| Input Item Name | Suggested input amount |
| Methanol (CH₃OH) | 1.9 to 2.1 kg |
| Water (H₂O, for reaction adjustment) | 0.05 to 0.1 kg |
| Solvents and other process substances Name | Approximate amount used (kg) | Cycloparametric rate |
| Catalyst (acidic catalyst, e.g. γ-Al₂O₃) | 0.01 to 0.02 kg | Almost 100%. |
| Water (for cooling and heating) | 3-5 kg | 80-95% (reused) |
| Type and amount of energy | Approximate consumption (kWh/kg) |
| electric power | 0.2 to 0.4 kWh |
| Thermal energy (steam or natural gas) | 1.5 to 2.0 kWh |
| Fuel Type | Approximate quantity (kg) |
| Natural gas or fuel oil (heat source) | 0.05 to 0.1 kg |
| 0 kg | CO₂ other than combustion
| Waste requiring treatment Name | Approximate quantity |
| Catalyst waste (after long-term use) | 0.001 to 0.005 kg |
| Process water (byproduct moisture) | 0.1 to 0.2 kg |
Sulfur Sludge Treatment
Sulfur sludge is a byproduct of the petroleum refining and natural gas refining processes, and is commonly generated after the treatment of H₂S (hydrogen sulfide). This sludge often contains sulfides and organic components that require appropriate treatment to reduce environmental impact.
A typical treatment method for sulfur sludge is to use a **Sulfur Recovery Unit (Claus Process)**. Below are approximate values for processing 1 kg of sulfur sludge using a method commonly used in industry.
Treatment of sulfur sludge by the Claus process
input
| Input Item Name | Suggested input amount |
| sulfur sludge | 1 kg (to be processed) |
| Air (for oxidation) | 0.8 to 1.0 kg (oxygen source) |
| Water (for cooling and cleaning) | 0.5 to 1.0 kg (process water) |
Solvents and other process substances
| name | Approximate amount used (kg) | Cycloparametric rate |
| Catalyst (Alumina catalyst) | 0.01 to 0.02 kg | Almost 100% (long-term use) |
| Water (for cooling) | 2 to 4 kg | 90-95% (circulation used) |
| Cleaning solvent (methanol or DME) | 0.05 to 0.1 kg | 70-85% (reused) |
Type and amount of energy
| Energy Type | Approximate consumption (kWh/kg) |
| electric power | 0.5 to 1.0 kWh |
| Thermal energy (natural gas or heavy oil) | 2.0 to 3.0 kWh |
Fuel type and quantity
| type | Approximate quantity (kg) |
| Natural gas or heavy oil (heat source) | 0.05 to 0.1 kg |
Other than combustion, CO₂
| Amount generated (kg) |
| 0 kg (no CO₂ is generated except by combustion) |
Waste requiring treatment
| name | Approximate quantity |
| Residue (unreacted sulfur or impurities) | 0.01 to 0.05 kg/kg Sludge |
| Cleaning waste (methanol or DME waste) | 0.01 to 0.05 kg/kg Sludge |
Process details and guidelines
- Main reaction (oxidation and reduction)
- Sulfur sludge is combusted or oxidized to produce H₂S, which is converted to sulfur in the Claus process. Air is supplied for oxidation and oxygen is used as the reaction source.
- Cooling and separation process
- The produced sulfur is cooled and separated as solid sulfur, and other impurities are removed. Since water for cooling is reused in the circulation system, the circulation rate is high, 90-95%, although the amount of water used is high.
- Treatment of by-products and unreacted materials
- Unreacted sulfur and other impurities (e.g., metal residues, heavy metals, etc.) may remain, which must ultimately be disposed of as waste.
- Disposal of Waste Liquid
- Methanol or DME (dimethyl ether) for cleaning is often reused, but long-term use requires treatment as waste liquid.
summary
| Input Item Name | Input amount guideline |
| sulfur sludge | 1 kg |
| Air (for oxidation) | 0.8 to 1.0 kg |
| Water (for cooling and cleaning) | 0.5 to 1.0 kg |
| Solvents and other process substances Name | Approximate amount used (kg) | Cycloparametric rate |
| Catalyst (Alumina catalyst) | 0.01 to 0.02 kg | Almost 100%. |
| Water (for cooling) | 2 to 4 kg | 90-95% of |
| Cleaning solvent (methanol or DME) | 0.05 to 0.1 kg | 70-85% (reused) |
| Type and amount of energy | Approximate consumption (kWh/kg) |
| electric power | 0.5 to 1.0 kWh |
| Thermal energy (natural gas or heavy oil) | 2.0 to 3.0 kWh |
| Fuel Type | Approximate quantity (kg) |
| Natural gas or heavy oil (heat source) | 0.05 to 0.1 kg |
| 0 kg | CO₂ other than combustion
| Waste requiring treatment Name | Approximate quantity |
| Residue (unreacted sulfur or impurities) | 0.01 to 0.05 kg |
| Cleaning effluent (methanol or DME effluent) | 0.01 to 0.05 kg |
rust inhibitor
Corrosion inhibitors are generally products based on** organic acid salts (phosphates, nitrates, carboxylates, etc.) or inorganic acid salts (zinc-based, chromate-based), but the manufacturing process and ingredients differ depending on the application. Here, we present a summary of inputs and approximate energy consumption based on the process for manufacturing 1 kg of zinc phosphate (Zinc Phosphate, Zn₃(PO₄)₂)**, one of the most common rust inhibitors.
Zinc Phosphate Production Process
Zinc phosphate is formed by the reaction of **phosphoric acid (H₃PO₄) with zinc sulfate (ZnSO₄)**. In this process, sulfuric acid (H₂SO₄) is generated as a byproduct along with the formation of zinc phosphate.
Reaction Formula: 3ZnSO4+2H3PO4→Zn3(PO4)2+3H2SO43\text{ZnSO}_4 + 2\text{H}_3\text{PO}_4 \rightarrow \text{Zn}_3(\text{PO}_4)_2 + 3\text{H}_2\text{SO}_ 43ZnSO4+2H3PO4→Zn3(PO4)2+3H2SO4
input
| Input Item Name | Suggested input amount |
| Phosphoric acid (H₃PO₄, 85% concentration) | 0.6-0.7 kg |
| Zinc sulfate (ZnSO₄, 98% concentration) | 0.8 to 1.0 kg |
| Water (for reaction adjustment) | 0.1 to 0.2 kg |
Solvents and other process substances
| name | Approximate amount used (kg) | Cycloparametric rate |
| Water (for cooling and reaction adjustment) | 3-5 kg | 80-90% (circulation used) |
| Catalyst (Acid catalyst) | 0.005 to 0.01 kg | Almost 100%. |
| Solvent for cleaning (e.g., methanol) | 0.05 to 0.1 kg | 70-80% (reused) |
Type and amount of energy
| Energy Type | Approximate consumption (kWh/kg) |
| electric power | 0.5 to 1.0 kWh |
| Thermal energy (steam) | 1.0 to 2.0 kWh |
Fuel type and quantity
| type | Approximate quantity (kg) |
| Natural gas or fuel oil (heat source) | 0.05 to 0.1 kg |
Other than combustion, CO₂
| Amount generated (kg) |
| 0.1 to 0.2 kg (reaction when using phosphoric acid) |
Waste requiring treatment
| name | Approximate quantity |
| Sulfuric acid (H₂SO₄, byproduct) | 0.3~0.5 kg/kg Rust inhibitor |
| Sludge (reaction byproduct) | 0.01~0.03 kg/kg Rust inhibitor |
Process details and guidelines
- Main reaction (reaction of phosphoric acid and zinc sulfate)
- Phosphoric acid (H₃PO₄) reacts with zinc sulfate (ZnSO₄) to produce zinc phosphate. This reaction yields sulfuric acid (H₂SO₄) as a byproduct, so treatment of the byproduct is necessary.
- Cooling and separation process
- The zinc phosphate produced is cooled, precipitated, and filtered. Water for cooling is often reused in the circulation system, with a circulation rate of 80-90%.
- Cleaning and drying process
- The product is dried and finally finished as a powder or crystalline zinc phosphate product. Thermal energy (steam or hot water) is consumed at this stage.
- Byproduct treatment
- Sulfuric acid produced during the reaction may be utilized in other processes or disposed of as waste if necessary.
summary
| Input Item Name | Suggested input amount |
| Phosphoric acid (H₃PO₄, 85% concentration) | 0.6-0.7 kg |
| Zinc sulfate (ZnSO₄, 98% concentration) | 0.8 to 1.0 kg |
| Water (for reaction adjustment) | 0.1 to 0.2 kg |
| Solvents and other process substances Name | Approximate amount used (kg) | Cycloparametric rate |
| Water (for cooling and reaction adjustment) | 3-5 kg | 80-90% (reused) |
| Catalyst (Acid catalyst) | 0.005 to 0.01 kg | Almost 100%. |
| Solvent for cleaning (e.g., methanol) | 0.05 to 0.1 kg | 70-80% of the total |
| Type and amount of energy | Approximate consumption (kWh/kg) |
| electric power | 0.5 to 1.0 kWh |
| Thermal energy (steam) | 1.0 to 2.0 kWh |
| Fuel Type | Approximate quantity (kg) |
| Natural gas or fuel oil (heat source) | 0.05 to 0.1 kg |
| 0.1 to 0.2 kg | CO₂ other than combustion
| Waste requiring treatment Name | Approximate quantity |
| Sulfuric acid (H₂SO₄, byproduct) | 0.3 to 0.5 kg |
| Sludge (reaction byproduct) | 0.01 to 0.03 kg |
oxygen scavenger
Deoxidizers are mainly used to remove oxygen from water or gases, and common types include** sodium sulfite (Na₂SO₃) and sodium hydride (NaH). Here are the inputs and approximate energy consumption for producing 1 kg of sodium sulfite (Na₂SO₃)**, the most commonly used type in industry.
Production process for sodium sulfite (Na₂SO₃)
Sodium sulfite is produced by the reaction of **sulfur dioxide (SO₂) with sodium hydroxide (NaOH)**. This reaction is an acid-base reaction and produces no specific byproducts.
Reaction equation:
SO2+2NaOH→Na2SO3+H2O\text{SO}_2 + 2\text{NaOH} \rightarrow \text{Na}_2\text{SO}_3 + \text{H}_2\text{O}SO2+2NaOH→Na2 SO3+H2O
input
| Input Item Name | Suggested input amount |
| Sulfur dioxide (SO₂) | 0.5 to 0.6 kg |
| Sodium hydroxide (NaOH) | 0.6-0.7 kg |
| Water (reactive use) | 0.1 to 0.2 kg |
Solvents and other process substances
| name | Approximate amount used (kg) | Cycloparametric rate |
| Catalysts (e.g., acid catalysts) | 0.005 to 0.01 kg | Almost 100% (long-term use) |
| Water (for cooling and heating) | 3-5 kg | 80-90% (circulation used) |
| Solvent for cleaning (e.g., methanol) | 0.05 to 0.1 kg | 70-85% (reused) |
Type and amount of energy
| Energy Type | Approximate consumption (kWh/kg) |
| electric power | 0.2 to 0.4 kWh |
| Thermal energy (steam) | 1.0 to 1.5 kWh |
Fuel type and quantity
| type | Approximate quantity (kg) |
| Natural gas or heavy oil (heat source) | 0.05 to 0.1 kg |
Other than combustion, CO₂
| Amount generated (kg) |
| 0.1-0.2 kg (CO₂ during NaOH production) |
Waste requiring treatment
| name | Approximate quantity |
| Sludge (impurity residue) | 0.01 to 0.02 kg/kg |
| Cleaning waste liquid (methanol, etc.) | 0.005 to 0.01 kg/kg |
Process details and guidelines
- Main reaction (reaction of SO₂ and NaOH)
- Sulfur dioxide (SO₂) reacts with sodium hydroxide (NaOH) to produce sodium sulfite. Water (H₂O) is produced as a byproduct, which requires cooling and separation after the reaction.
- Cooling and separation process
- The product is cooled, precipitated, and filtered. Since water for cooling is reused in the circulation system, the circulation rate is 80-90%, although the amount of water used is high.
- Cleaning and drying process
- The product is dried and finally finished as a powder or crystalline sodium sulfite product. Thermal energy (steam or hot water) is consumed at this stage.
- Treatment of by-products and impurities
- Byproducts formed during the reaction and impurities remaining after the reaction (e.g., metal residues, unreacted materials) must be separated as sludge and disposed of as waste.
summary
| Input Item Name | Suggested input amount |
| Sulfur dioxide (SO₂) | 0.5 to 0.6 kg |
| Sodium hydroxide (NaOH) | 0.6-0.7 kg |
| Water (reactive use) | 0.1 to 0.2 kg |
| Solvents and other process substances Name | Approximate amount used (kg) | Cycloparametric rate |
| Catalyst (Acid catalyst) | 0.005 to 0.01 kg | Almost 100%. |
| Water (for cooling and heating) | 3-5 kg | 80-90% (reused) |
| Solvent for cleaning (e.g., methanol) | 0.05 to 0.1 kg | 70-85% of the total |
| Type and amount of energy | Approximate consumption (kWh/kg) |
| electric power | 0.2 to 0.4 kWh |
| Thermal energy (steam) | 1.0 to 1.5 kWh |
| Fuel Type | Approximate quantity (kg) |
| Natural gas or heavy oil (heat source) | 0.05 to 0.1 kg |
| 0.1 to 0.2 kg | CO₂ other than combustion
| Waste requiring treatment Name | Quantity standard |
| Sludge (impurity residue) | 0.01 to 0.02 kg |
| Cleaning waste liquid (methanol, etc.) | 0.005 to 0.01 kg |
gas field gas
Gas Field Gas (Gas Field Gas) is gas extracted from underground gas fields where natural gas is the main component, usually methane (CH₄), and may contain components such as ethane, propane, butane, nitrogen, carbon dioxide (CO₂), and hydrogen sulfide (H₂S). Mining for this gas involves processes such as drilling, pressure control, extraction, and initial processing (removal of impurities). Below is a rough estimate of the inputs and energy consumption for mining 1 kg of gas field gas.
Gas Field Gas Mining Process
The mining process generally consists of the following steps
- Drilling: drilling of gas fields and installation of mining equipment
- Extraction: extraction of underground gas, pressure control, pipeline transportation of gas
- Initial treatment: removal of impurities such as H₂S and CO₂.
input
| Input Item Name | Suggested input amount |
| Drilling fluid (drilling mud) | 0.2 to 0.3 kg (when drilling) |
| chemical additive | 0.05-0.1 kg (flocculant, pH adjuster, etc.) |
Solvents and other process substances
| name | Approximate amount used (kg) | Cycloparametric rate |
| Water (for drilling) | 5-10 kg (for drilling) | 70-90% (partially reused) |
| Amine solution (for CO₂ removal) | 0.05 to 0.1 kg | 90-95% of |
| Glycol (for dehydration) | 0.05 to 0.1 kg | 80-90% (reused) |
Type and amount of energy
| Energy Type | Approximate consumption (kWh/kg) |
| Electric power (for pumps and compressors) | 0.1 to 0.3 kWh |
| Thermal energy (for gas processing) | 0.2 to 0.4 kWh |
Fuel type and quantity
| type | Approximate quantity (kg) |
| Natural gas (for own consumption) | 0.01 to 0.05 kg |
Other than combustion, CO₂
| Amount generated (kg) |
| 0.02 to 0.05 kg (gas emissions during drilling) |
Waste requiring treatment
| name | Approximate quantity |
| Drilling Mud (Drill Cuttings) | 0.01 to 0.02 kg/kg |
| Spent amine solution | 0.005 to 0.01 kg/kg |
| Spent glycol solution | 0.005 to 0.01 kg/kg |
Process details and guidelines
- Drilling and extraction
- Mining from underground gas fields requires initial drilling and well maintenance. Drilling fluids (drilling fluids) and chemical additives are used for drilling.
- Gas extraction and compression
- The extracted gas must be compressed for pipeline transport and initial processing. This process involves power consumption by pumps and compressors.
- Initial processing and impurity removal
- The collected gas often contains H₂S and CO₂, which are removed using an amine solution. Glycol is used for dehydration and reused in circulation.
- Waste Disposal
- Waste materials such as drill cuttings and spent amine and glycol solutions are generated during drilling. These must go through a waste treatment process.
summary
| Input Item Name | Suggested input amount |
| Drilling Fluid (Drilling Mud) | 0.2 to 0.3 kg |
| chemical additive | 0.05 to 0.1 kg |
| Solvents and other process substances Name | Approximate amount used (kg) | Cycloparametric rate |
| Water (for drilling) | 5-10 kg | 70-90% (partially reused) |
| Amine solution (for CO₂ removal) | 0.05 to 0.1 kg | 90-95% of |
| Glycol (for dehydration) | 0.05 to 0.1 kg | 80-90% of the total |
| Type and amount of energy | Approximate consumption (kWh/kg) |
| electric power | 0.1 to 0.3 kWh |
| Thermal energy (for gas processing) | 0.2 to 0.4 kWh |
| Fuel Type | Approximate quantity (kg) |
| Natural gas (for own consumption) | 0.01 to 0.05 kg |
| 0.02 to 0.05 kg | CO₂ other than combustion
| Waste requiring treatment Name | Approximate quantity |
| Drilling Mud (Drill Cuttings) | 0.01 to 0.02 kg |
| Spent amine solution | 0.005 to 0.01 kg |
| Spent glycol solution | 0.005 to 0.01 kg |
glycol
Glycol (Ethylene Glycol, C₂H₄(OH)₂) and Propylene Glycol (Propylene Glycol, C₃H₆(OH)₂) are mainly produced industrially and are widely used as coolants and dehydrating agents. Here are the inputs and approximate energy consumption for the production of 1 kg of glycol, based on a typical **Ethylene Glycol (EG)** production process.
Ethylene glycol (EG) production process
Ethylene glycol is generally produced by the reaction of **ethylene oxide (EO) with water (H₂O)**. This process is carried out in the presence of an acid catalyst, and the yield of ethylene glycol can be increased by adjusting the reaction conditions.
Reaction formula:
C2H4O+H2O→C2H4(OH)2\text{C}_2\text{H}_4\text{O} + \text{H}_2\text{O} \rightarrow \text{C}_2\text{H}_4(\text{OH})_2C2H 4O+H2O→C2H4(OH)2
input
| Input Item Name | Suggested input amount |
| Ethylene oxide (EO) | 0.6 to 0.7 kg |
| Water (H₂O) | 0.5 to 0.7 kg |
Solvents and other process substances
| name | Approximate amount used (kg) | Cycloparametric rate |
| Catalyst (Acid catalyst) | 0.005 to 0.01 kg | Almost 100% (long-term use) |
| Water (for cooling and heating) | 4-6 kg | 85-95% (circulation use) |
| Solvent for cleaning (e.g., methanol) | 0.05 to 0.1 kg | 70-85% (reused) |
Type and amount of energy
| Energy Type | Approximate consumption (kWh/kg) |
| electric power | 0.3 to 0.5 kWh |
| Thermal energy (steam) | 2.0 to 2.5 kWh |
Fuel type and quantity
| type | Approximate quantity (kg) |
| Natural gas or fuel oil (heat source) | 0.05 to 0.1 kg |
Other than combustion, CO₂
| Amount generated (kg) |
| 0.1 to 0.2 kg (when generating EO) |
Waste requiring treatment
| name | Approximate quantity |
| Byproducts (e.g., diethylene glycol) | 0.02~0.05 kg/kg EG |
| Sludge (reaction residue) | 0.01 to 0.02 kg/kg EG |
| Cleaning waste liquids (e.g., methanol waste) | 0.005~0.01 kg/kg EG |
Process details and guidelines
- Main reaction (reaction of ethylene oxide and water)
- Ethylene glycol (EG) is produced by reacting ethylene oxide (EO) with water (H₂O). Small amounts of diethylene glycol (DEG) and triethylene glycol (TEG) may be produced as byproducts.
- Cooling and separation process
- The product is cooled and the mixture of ethylene glycol and water is separated. Water for cooling is used in the circulation system, resulting in a high circulation rate (85-95%), although the amount used is high.
- Purification and drying
- The resulting ethylene glycol is purified to remove unreacted ethylene oxide and water. Heat energy (steam) is used during the purification process, which also consumes electricity.
- Byproduct treatment
- Small amounts of diethylene glycol (DEG) and triethylene glycol (TEG) are formed during the reaction, which are either re-purified or disposed of as waste.
summary
| Input Item Name | Suggested input amount |
| Ethylene oxide (EO) | 0.6-0.7 kg |
| Water (H₂O) | 0.5 to 0.7 kg |
| Solvents and other process substances Name | Approximate amount used (kg) | Cycloparametric rate |
| Catalyst (Acid catalyst) | 0.005 to 0.01 kg | Almost 100%. |
| Water (for cooling and heating) | 4-6 kg | 85-95% (reused) |
| Solvent for cleaning (e.g., methanol) | 0.05 to 0.1 kg | 70-85% of the total |
| Type and amount of energy | Approximate consumption (kWh/kg) |
| electric power | 0.3 to 0.5 kWh |
| Thermal energy (steam) | 2.0 to 2.5 kWh |
| Fuel Type | Approximate quantity (kg) |
| Natural gas or fuel oil (heat source) | 0.05 to 0.1 kg |
| 0.1 to 0.2 kg | CO₂ other than combustion
| Waste requiring treatment Name | Approximate quantity |
| Byproducts (DEG, TEG, etc.) | 0.02 to 0.05 kg |
| Sludge (reaction residue) | 0.01 to 0.02 kg |
| Cleaning waste liquids (e.g., methanol waste) | 0.005 to 0.01 kg |
methanol
Methanol (CH₃OH) is often produced mainly from natural gas (methane, CH₄). In industrial terms, the most common process is “steam reforming of methane to produce syngas (H₂ and CO), followed by methanol synthesis. Below are the inputs and approximate energy consumption for producing 1 kg of methanol.
Methanol Production Process
The manufacturing process is largely divided into the following steps
- Natural gas reforming: Methane is reformed with steam at high temperature to produce syngas (H₂ and CO).
- Methanol synthesis: Synthesis gas is reacted in the presence of a catalyst to produce methanol.
- Separation and purification: Separation and purification of unreacted gas and by-products from the product.
input
| Input Item Name | Suggested input amount |
| Natural gas (methane, CH₄) | 0.5 to 0.6 kg |
| Water (H₂O, steam for reforming) | 0.4 to 0.5 kg |
Solvents and other process substances
| name | Approximate amount used (kg) | Cycloparametric rate |
| Catalyst (copper-based catalyst) | 0.01 to 0.02 kg | Almost 100% (long-term use) |
| Cooling water (for cooling and separation) | 5-10 kg | 90-95% (circulation used) |
| Cleaning solvent (methanol) | 0.05 to 0.1 kg | 80-90% (reused) |
Type and amount of energy
| Energy Type | Approximate consumption (kWh/kg) |
| Electric power (for pumps and compressors) | 0.5 to 1.0 kWh |
| Thermal energy (steam, natural gas combustion) | 2.5 to 3.0 kWh |
Fuel type and quantity
| type | Approximate quantity (kg) |
| Natural gas (for own consumption, heat source) | 0.05 to 0.1 kg |
Other than combustion, CO₂
| Amount generated (kg) |
| 0.1-0.2 kg (byproduct of steam reforming) |
Waste requiring treatment
| name | Approximate quantity |
| Catalyst waste (after long-term use) | 0.001 to 0.005 kg |
| Cleaning waste liquids (e.g., methanol waste) | 0.005 to 0.01 kg |
| Separation residue (heavy hydrocarbons, impurities) | 0.01 to 0.02 kg/kg Methanol |
Process details and guidelines
- Natural gas reforming and synthesis gas generation
- Methane (CH₄) is fed into the reformer along with steam to produce **synthesis gas (H₂ and CO)** at a high temperature of approximately 800 to 1000°C. This reaction also produces some CO₂ as a byproduct.
Reforming reaction formula:
CH4+H2O→CO+3H2\text{CH}_4 + \text{H}_2\text{O} \rightarrow \text{CO} + 3\text{H}_2CH4+H2O→CO+3H2
Side reaction equation:
CO+H2O→CO2+H2\text{CO} + \text{H}_2\text{O} \rightarrow \text{CO}_2 + \text{H}_2CO+H2O→CO2+H2
- Methanol Synthesis
- Synthesis gas generated (H₂
= 2:1) react with a catalyst (copper-based catalyst) at a temperature of about 300°C to produce methanol.
- Methanol synthesis reaction formula:
CO+2H2→CH3OH\text{CO} + 2\text{H}_2 \rightarrow \text{CH}_3\text{OH}CO+2H2→CH3OH - Cooling and Separation
- The product is cooled and unreacted gas and byproducts are separated. Unreacted gas is recirculated to separate and purify methanol.
- Control of by-products and catalysts
- Catalyst replacement is necessary when catalyst activity declines after long-term use. Catalyst waste is chemically treated and reused or disposed of.
- Waste and Environmental Impact Management
- CO₂ produced during the reforming process may be recovered as a byproduct, but some may be released, requiring environmental management.
summary
| Input Item Name | Suggested input amount |
| Natural gas (methane, CH₄) | 0.5 to 0.6 kg |
| Water (H₂O, steam for reforming) | 0.4 to 0.5 kg |
| Solvents and other process substances Name | Approximate amount used (kg) | Cycloparametric rate |
| Catalyst (copper-based catalyst) | 0.01 to 0.02 kg | Almost 100% (long-term use) |
| Cooling water (for cooling and separation) | 5-10 kg | 90-95% of |
| Cleaning solvent (methanol) | 0.05 to 0.1 kg | 80-90% of the total |
| Type and amount of energy | Approximate consumption (kWh/kg) |
| electric power | 0.5 to 1.0 kWh |
| Thermal energy (steam, natural gas combustion) | 2.5 to 3.0 kWh |
| Fuel Type | Approximate quantity (kg) |
| Natural gas (for own consumption, heat source) | 0.05 to 0.1 kg |
| 0.1 to 0.2 kg | CO₂ other than combustion
| Waste requiring treatment Name | Approximate quantity |
| Catalyst waste (after long-term use) | 0.001 to 0.005 kg |
| Cleaning waste liquids (e.g., methanol waste) | 0.005 to 0.01 kg |
| Separation residue (heavy hydrocarbons, impurities) | 0.01 to 0.02 kg |
deionized water
Deionized water (DI Water) is usually produced industrially by reverse osmosis (RO), ion exchange resin, or a combination of both. Below is a summary of the inputs and energy consumption for producing 1 kg of deionized water based on typical processes for producing deionized water using reverse osmosis and ion exchange resin methods.
Deionized water production process
The manufacturing process consists of the following steps
- Raw water pretreatment: Coarse filtration and activated carbon filters remove large impurities in the water.
- Reverse Osmosis (RO): Water is passed through a semipermeable membrane under pressure to remove ions and impurities.
- Purification by ion exchange resin: Water treated by RO is further purified to reduce the final ion concentration to the limit.
input
| Input Item Name | Suggested input amount |
| Raw water (tap water) | 1.2 to 3.0 kg |
Solvents and other process substances
| name | Approximate amount used (kg) | Cycloparametric rate |
| ion exchange resin | 0.001 to 0.002 kg | Almost 100% (long-term use) |
| Activated carbon filter | 0.01 to 0.05 kg | 50-70% (recyclable) |
| Acid (for resin regeneration, e.g., sulfuric acid) | 0.005 to 0.01 kg | 80-90% (recycled and reused) |
| Alkaline agent (for regeneration, e.g., caustic soda) | 0.005 to 0.01 kg | 80-90% (recycled and reused) |
Type and amount of energy
| Energy Type | Approximate consumption (kWh/kg) |
| Power (for RO and pumps) | 0.5 to 1.0 kWh |
| Thermal energy (for regenerative treatment) | 0.1 to 0.2 kWh |
Fuel type and quantity
| type | Approximate quantity (kg) |
| Natural gas or electricity (for heating) | 0.01 to 0.02 kg |
Other than combustion, CO₂
| Amount generated (kg) |
| 0 kg (usually no CO₂ is generated) |
Waste requiring treatment
| name | Approximate quantity |
| Waste resin (ion exchange resin) | 0.001~0.002 kg/kg |
| Used activated carbon filter | 0.01 to 0.02 kg/kg |
| Reclaimed waste liquids (acid and alkali waste liquids) | 0.01 to 0.05 kg/kg |
Process details and guidelines
- Raw water pretreatment
- Raw water (usually tap water) used to produce deionized water is pretreated to remove large particles, chlorine, and organic matter. Activated carbon filters and coarse filtration filters are used, which are periodically regenerated.
- Reverse Osmosis Treatment (RO)
- Raw water is passed through a semi-permeable membrane (reverse osmosis membrane) using a high-pressure pump to remove dissolved ions and solutes. Energy (electricity) is consumed in this process, and approximately 20-40% of the water is removed as wastewater, resulting in a low circulation rate.
- Final purification by ion exchange resin
- Water treated by reverse osmosis is passed through an ion exchange resin to remove residual ions and increase final purity. The resin can be used for long periods of time, but is periodically regenerated with acid and alkaline solutions.
- Reclamation and waste management
- Acids and alkalis used in the regeneration process are often circulated for use, but some are discharged as liquid waste. Waste may also be generated due to deterioration of activated carbon and ion exchange resins.
summary
| Input Item Name | Suggested input amount |
| Raw water (tap water) | 1.2 to 3.0 kg |
| Solvents and other process substances Name | Approximate amount used (kg) | Cycloparametric rate |
| ion exchange resin | 0.001 to 0.002 kg | Almost 100%. |
| Activated carbon filter | 0.01 to 0.05 kg | 50-70% (recyclable) |
| Acid (e.g., sulfuric acid) | 0.005 to 0.01 kg | 80-90% (reused) |
| Alkaline agent (e.g., caustic soda) | 0.005 to 0.01 kg | 80-90% (reused) |
| Type and amount of energy | Approximate consumption (kWh/kg) |
| Power (for RO and pumps) | 0.5 to 1.0 kWh |
| Thermal energy (for regenerative treatment) | 0.1 to 0.2 kWh |
| Fuel Type | Approximate quantity (kg) |
| Natural gas or electricity (for heating) | 0.01 to 0.02 kg |
| 0 kg | CO₂ other than combustion
| Waste requiring treatment Name | Approximate quantity |
| Waste resin (ion exchange resin) | 0.001 to 0.002 kg |
| Used activated carbon filter | 0.01 to 0.02 kg |
| Reclaimed waste liquids (acid and alkali waste liquids) | 0.01 to 0.05 kg |
This table summarizes the inputs, energy consumption, waste generation, and recirculation rate of process materials for the production of 1 kg of deionized water.
Ion exchange resin degradation varies greatly depending on the type of resin, the quality of the water to be treated (especially the amount of hardness components and organic matter in the water), and operating conditions (flow rate, temperature, pH, etc.). Typical industrial ion exchange resins usually have a service life of 1 to 3 years, during which time deteriorated resins must be replaced or discarded. This can be quantified as the amount of resin degradation per 1 kg of water, which can be used as a guide as follows.
Approximate amount of ion exchange resin degradation
The estimated amount of degradation for treating 1 kg of water is estimated by considering the annual usage and lifetime of the resin.
Calculation of deterioration rate
- Total amount of resin: Typically, about 100-300 kg of resin per cubic meter (1 m³) is used, for example, although the amount of industrial resin filled will depend on operating conditions and the size of the equipment.
- Resin life: Based on a 1-3 year life expectancy, the amount of resin degraded per year is estimated to be about 10-20% of the total amount (depending on the frequency of regeneration and operating conditions).
This translates to per kg of water treatment:
Approximate amount of resin degradation (per kg of water treated)
- 0.001-0.003 g/kg water
This value should be considered as a reference value only, as it varies depending on the type of resin (cation exchange resin or anion exchange resin), water quality (high hardness water or water with high organic matter), and operating conditions (regeneration frequency, flow rate, and temperature).
Considerations based on more detailed conditions
- Effect of regeneration cycles: If regeneration operations are performed frequently, physical wear is likely to progress and the amount of degradation will increase.
- Resin type: Specialty resins, especially those suitable for organic removal or use in high temperature conditions, may deteriorate faster than ordinary resins.
To more accurately estimate the amount of resin degradation, it is necessary to consider the service life and replacement frequency based on the composition of the treated water and operating conditions. It is recommended that calculations be based on the specific conditions of the plant and data on the ion exchange resins used.
zinc phosphate
Zinc phosphate (Zinc Phosphate, Zn₃(PO₄)₂) is a compound used primarily as a rust inhibitor and coating material. The common production process involves the reaction of **phosphoric acid (H₃PO₄) with zinc sulfate (ZnSO₄)**. This process produces sulfuric acid (H₂SO₄) as a byproduct along with zinc phosphate.
Below is a rough estimate of the inputs and energy consumption for producing 1 kg of zinc phosphate.
Zinc Phosphate Production Process
Reaction Formula: 3ZnSO4+2H3PO4→Zn3(PO4)2+3H2SO43\text{ZnSO}_4 + 2\text{H}_3\text{PO}_4 \rightarrow \text{Zn}_3(\text{PO}_4)_2 + 3\text{H}_2\text{SO}_ 43ZnSO4+2H3PO4→Zn3(PO4)2+3H2SO4
In this reaction, 3 moles of zinc sulfate react with 2 moles of phosphoric acid to form zinc phosphate and sulfuric acid.
input
| Input Item Name | Suggested input amount |
| Phosphoric acid (H₃PO₄, 85% concentration) | 0.4 to 0.5 kg |
| Zinc sulfate (ZnSO₄, 98% concentration) | 0.6 to 0.7 kg |
| Water (for reaction adjustment and dilution) | 0.1 to 0.2 kg |
Solvents and other process substances
| name | Approximate amount used (kg) | Cycloparametric rate |
| Catalyst (acidic or basic catalyst) | 0.005 to 0.01 kg | Almost 100% (long-term use) |
| coolant | 5-10 kg | 80-90% (circulation used) |
| Solvent for cleaning (e.g., methanol) | 0.05 to 0.1 kg | 70-85% (reused) |
Type and amount of energy
| Energy Type | Approximate consumption (kWh/kg) |
| electric power | 0.2 to 0.4 kWh |
| Thermal energy (steam or natural gas) | 1.5 to 2.0 kWh |
Fuel type and quantity
| type | Approximate quantity (kg) |
| Natural gas or fuel oil (heat source) | 0.05 to 0.1 kg |
Other than combustion, CO₂
| Amount generated (kg) |
| 0.1-0.2 kg (CO₂ generation in reaction) |
Waste requiring treatment
| name | Approximate quantity |
| Byproducts (sulfuric acid, H₂SO₄) | 0.5-0.7 kg/kg Zn₃(PO₄)₂ |
| Sludge (reaction byproduct) | 0.01 to 0.03 kg/kg Zn₃(PO₄)₂ |
| Cleaning waste liquids (e.g., methanol waste) | 0.01-0.02 kg/kg Zn₃(PO₄)₂ |
Process details and guidelines
- Main reaction (reaction of phosphoric acid and zinc sulfate)
- The reaction of phosphoric acid (H₃PO₄) with zinc sulfate (ZnSO₄) produces zinc phosphate (Zn₃(PO₄)₂). This reaction yields sulfuric acid (H₂SO₄) as a byproduct.
- Cooling and separation process
- The zinc phosphate produced is cooled, precipitated, and then filtered for separation. Since water for cooling is reused in the circulation system, the circulation rate is 80-90%, although the amount used is high.
- Cleaning and drying process
- The product is washed with water and then dried to produce the final product. This stage consumes a lot of thermal energy and electricity.
- Byproduct treatment
- Sulfuric acid, a byproduct formed during the reaction, may be used in other chemical processes, but usually requires neutralization or other treatment.
summary
| Input Item Name | Suggested input amount |
| Phosphoric acid (H₃PO₄, 85% concentration) | 0.4 to 0.5 kg |
| Zinc sulfate (ZnSO₄, 98% concentration) | 0.6 to 0.7 kg |
| Water (for reaction adjustment and dilution) | 0.1 to 0.2 kg |
| Solvents and other process substances Name | Approximate amount used (kg) | Cycloparametric rate |
| Catalyst (acidic or basic catalyst) | 0.005 to 0.01 kg | Almost 100%. |
| coolant | 5-10 kg | 80-90% (reused) |
| Solvent for cleaning (e.g., methanol) | 0.05 to 0.1 kg | 70-85% of the total |
| Type and amount of energy | Approximate consumption (kWh/kg) |
| electric power | 0.2 to 0.4 kWh |
| Thermal energy (steam) | 1.5 to 2.0 kWh |
| Fuel Type | Approximate quantity (kg) |
| Natural gas or fuel oil (heat source) | 0.05 to 0.1 kg |
| 0.1 to 0.2 kg | CO₂ other than combustion
| Waste requiring treatment Name | Approximate quantity |
| Byproducts (sulfuric acid, H₂SO₄) | 0.5 to 0.7 kg |
| Sludge (reaction byproduct) | 0.01 to 0.03 kg |
| Cleaning waste liquids (e.g., methanol waste) | 0.01 to 0.02 kg |
natural gas drilling
There are three main types of drilling fluids widely used in industrial natural gas drilling: Water-Based Fluids, Oil-Based Fluids, and **Synthetic-Based Fluids**. Oil-Based Fluids, and **Synthetic-Based Fluids**. The share percentages of these drilling fluids are as follows
Share composition of drilling fluids (as of 2022)
- Water-Based Fluids: approx. 52
- Oil-Based Fluids: approx. 36
- Synthetic-Based Fluids: approx. 12
The total market share of these three drilling fluids is 100%.
Characteristics of various drilling fluids
- Water-based drilling fluids are often used, especially for onshore drilling, because they are cost-effective and have minimal environmental impact.
- Oil-based drilling fluids are commonly used in difficult drilling conditions and high-temperature environments because of their excellent lubricity and ability to reduce tool wear.
- Synthetic base drilling fluids have a low environmental impact and are increasingly used in special conditions (deep water and high pressure environments).
sodium sulfite
Sodium sulfite (Sodium Sulfite, Na₂SO₃) is a compound used primarily in industrial applications (such as oxygen scavengers and bleaching agents). It is typically produced by the reaction of sulfur dioxide (SO₂) with sodium hydroxide (NaOH). In this process, sulfur dioxide is absorbed by an aqueous sodium hydroxide solution to produce sodium sulfite.
The following is a summary of the inputs and energy consumption for producing 1 kg of sodium sulfite.
Production process for sodium sulfite
Main reaction equation:
SO2+2NaOH→Na2SO3+H2O\text{SO}_2 + 2\text{NaOH} \rightarrow \text{Na}_2\text{SO}_3 + \text{H}_2\text{O}SO2+2NaOH→Na2 SO3+H2O
This reaction produces 1 mole of sodium sulfite from 1 mole of sulfur dioxide and 2 moles of sodium hydroxide, with water as a byproduct.
Approximate Inputs and Energy
Input power
| (data) item | Approximate quantity (kWh/kg Na₂SO₃) |
| electric power | 0.2 to 0.5 kWh |
Input fuel
| type | Approximate quantity (kg) |
| Natural gas or fuel oil (for heating) | 0.05 to 0.1 kg |
Other than combustion, CO₂
| Approximate amount generated (kg CO₂/kg Na₂SO₃) |
| 0.1 to 0.2 kg |
input
| name | Approximate input amount (kg/kg Na₂SO₃) |
| Sulfur dioxide (SO₂) | 0.4 to 0.5 kg |
| Sodium hydroxide (NaOH) | 0.5 to 0.6 kg |
| Water (H₂O) | 0.1 to 0.2 kg |
Solvents and other process substances
| name | Approximate amount used (kg) | Cycloparametric rate |
| Water (for cooling and cleaning) | 2 to 3 kg | 85-95% (circulation use) |
| Solvent for cleaning (e.g., methanol) | 0.05 to 0.1 kg | 70-85% (reused) |
Waste requiring treatment
| name | Approximate amount (kg/kg Na₂SO₃) |
| Unreacted material and sludge | 0.01 to 0.02 kg |
| Cleaning waste liquids (e.g., methanol waste) | 0.005 to 0.01 kg |
Process details and guidelines
- Main reaction (reaction of SO₂ and NaOH)
- Sulfur dioxide (SO₂) reacts with sodium hydroxide (NaOH) to produce sodium sulfite. Water (H₂O) is produced as a byproduct of this reaction.
- Cooling and separation process
- After the reaction, the product is cooled to precipitate sodium sulfite and then filtered. Cooling water is reused in the circulation system, so although the amount used is high, the circulation rate is high (85-95%) and can be reused.
- Cleaning and drying process
- The product is dried and finally finished as a powder or crystalline sodium sulfite product. This stage consumes thermal energy (steam and hot water) and also uses electricity.
- Waste Disposal
- A small amount of byproducts and sludge are generated during the reaction, which can be disposed of properly or reused in other processes.
summary
| (data) item | Approximate values |
| Input power | 0.2 to 0.5 kWh |
| Input fuel (natural gas or fuel oil) | 0.05 to 0.1 kg |
| Other than combustion, CO₂ | 0.1 to 0.2 kg |
| Input Item Name | Approximate input amount (kg) |
| Sulfur dioxide (SO₂) | 0.4 to 0.5 kg |
| Sodium hydroxide (NaOH) | 0.5 to 0.6 kg |
| Water (H₂O) | 0.1 to 0.2 kg |
| Solvents and other process substances Name | Approximate amount used (kg) | Cycloparametric rate |
| Water (for cooling and cleaning) | 2 to 3 kg | 85-95% (reused) |
| Solvent for cleaning (e.g., methanol) | 0.05 to 0.1 kg | 70-85% of the total |
| Waste requiring treatment Name | Approximate quantity (kg) |
| Unreacted material and sludge | 0.01 to 0.02 kg |
| Cleaning waste liquids (e.g., methanol waste) | 0.005 to 0.01 kg |
coagulant
The three substances with the highest share of industrially used flocculants (Coagulants) are
- Aluminum Sulfate (Al₂(SO₄)₃)
- Market share: approx. 40
- Aluminum sulfate is the most commonly used inorganic flocculant, effectively flocculating suspended particles in water and used in a wide range of applications [99].
- Poly DADMAC (Polydiallyldimethylammonium chloride)
- Market share: approx. 30
- It is an organic coagulant, characterized by its high coagulation effect and operability. In particular, it is used in a variety of water quality conditions because it does not depend much on the pH of the water [98].
- Ferric Chloride (FeCl₃)
- Market share: approx. 30
- It is widely used as an inorganic coagulant and is particularly suitable for water with high turbidity and water containing difficult-to-treat organic matter [99].
These three flocculants have a combined overall market share of 100%. When selecting a flocculant, it is important to consider its characteristics, water quality, and cost-effectiveness.
pH adjuster
The main substances industrially used as pH adjusters for water treatment and their share percentages are as follows
Top three pH adjusters
- Sulfuric Acid, H₂SO₄
- Market share: 40
- Sulfuric acid is the most commonly used pH adjuster and is used in many water treatment applications because it effectively adjusts the pH of water and can easily manage acidic conditions.
- Caustic soda (sodium hydroxide, NaOH)
- Market share: 35
- Caustic soda is widely used as an alkaline pH adjuster, especially for neutralizing acidic water and stabilizing pH.
- Sodium carbonate (Na₂CO₃)
- Market share: 25
- Sodium carbonate is more mildly alkaline than caustic soda, making it suitable for fine-tuning pH and managing pH balance in certain chemical processes.
The sum of the share percentages of these three pH adjusters is 100% (40% + 35% + 25%). Depending on the application and water quality, a combination of these substances may be used, but the above substances are the most universally used in the pH adjuster market.
ethylene oxide
Ethylene oxide (EO) is an important chemical produced primarily by oxidizing ethylene. The process of reacting ethylene with oxygen is widely used in industry. This process uses silver oxide (Ag) as a catalyst to control the reaction and maximize ethylene oxide production. Below is a summary of the inputs, energy consumption, and approximate waste for the production of 1 kg of ethylene oxide.
Ethylene oxide production process
The main reaction equations are as follows
C2H4+12O2→C2H4O\text{C}_2\text{H}_4 + \frac{1}{2}\text{O}_2 \rightarrow \text{C}_2\text{H}_4\text{O}C2H4+21 O2→C2H4O
This reaction produces 1 mole of ethylene oxide (C₂H₄O) from 1 mole of ethylene (C₂H₄) and 1/2 mole of oxygen (O₂). Small amounts of carbon dioxide (CO₂) and water (H₂O) may be generated as byproducts.
Inputs and energy required for ethylene oxide production
Input power
| (data) item | Approximate quantity (kWh/kg EO) |
| Electric power (for compression and pumps) | 0.3 to 0.5 kWh |
Input fuel
| type | Approximate quantity (kg) |
| Natural gas (fuel for heating) | 0.1 to 0.2 kg |
Other than combustion, CO₂
| Approximate amount generated (kg CO₂/kg EO) |
| 0.05 to 0.1 kg |
input
| name | Approximate amount of input (kg/kg EO) |
| Ethylene (C₂H₄) | 0.6 to 0.7 kg |
| Oxygen (O₂) | 0.2 to 0.3 kg |
Solvents and other process substances
| name | Approximate amount used (kg) | Cycloparametric rate |
| Catalyst (silver oxide) | 0.005 to 0.01 kg | Almost 100% (long-term use) |
| coolant | 5-10 kg | 80-90% (circulation used) |
| Cleaning solvent (e.g., methanol) | 0.01 to 0.05 kg | 70-80% (reused) |
Waste requiring treatment
| name | Approximate quantity (kg/kg EO) |
| Byproducts (CO₂ and H₂O) | 0.05 to 0.1 kg |
| Catalyst waste (silver oxide waste) | 0.001 to 0.003 kg |
| Cleaning waste liquids (e.g., methanol waste) | 0.005 to 0.01 kg |
Process details and guidelines
- Main reaction (oxidation reaction of ethylene and oxygen)
- Ethylene (C₂H₄) and oxygen (O₂) react using a silver oxide catalyst to produce ethylene oxide (C₂H₄O). This reaction takes place at 250-300°C and 10-30 bar pressure conditions. Controlled reactions increase the yield of ethylene oxide and minimize the formation of byproducts.
- Cooling and separation process
- The product is cooled, and unreacted ethylene and oxygen are separated and recirculated. Since a large amount of cooling water is used for cooling, the efficiency of the circulation system is critical.
- Purification and drying
- The ethylene oxide produced is purified in a refining process to remove impurities and finally separated as high-purity ethylene oxide. This process consumes electrical and thermal energy.
- Control of byproducts
- Small amounts of carbon dioxide (CO₂) and water (H₂O) are produced during the reaction, which require waste gas treatment and wastewater treatment.
summary
| (data) item | Approximate values |
| Input power | 0.3 to 0.5 kWh |
| Input fuel (natural gas) | 0.1 to 0.2 kg |
| Other than combustion, CO₂ | 0.05 to 0.1 kg |
| Input Item Name | Approximate input amount (kg) |
| Ethylene (C₂H₄) | 0.6 to 0.7 kg |
| Oxygen (O₂) | 0.2 to 0.3 kg |
| Solvents and other process substances Name | Approximate amount used (kg) | Cycloparametric rate |
| Catalyst (silver oxide) | 0.005 to 0.01 kg | Almost 100% (reused) |
| coolant | 5-10 kg | 80-90% of the total |
| Cleaning solvent (methanol) | 0.01 to 0.05 kg | 70-80% of the total |
| Waste requiring treatment Name | Approximate quantity |
| Byproducts (CO₂ and H₂O) | 0.05 to 0.1 kg |
| Catalyst waste (silver oxide waste) | 0.001 to 0.003 kg |
| Cleaning waste liquids (e.g., methanol waste) | 0.005 to 0.01 kg |
Sludge treatment
Aqueous Sludge is a mixture of liquids and solids generated primarily from processes such as industrial wastewater treatment, metal processing, surface treatment, and paper manufacturing. Commonly used treatment methods include dewatering, drying, and incineration. Below is a rough estimate of the input and energy consumption for treating 1 kg of aqueous sludge.
Treatment process for aqueous sludge
Typical treatment processes are as follows
- Dehydration (filtration or centrifugation):
- Water is separated from the sludge to increase the solids content. Electricity and flocculants consumed during this stage are required.
- Drying process:
- After dewatering, the sludge is further dried to reduce the moisture content and facilitate incineration. Thermal energy is required at this stage.
- Incineration or solidification:
- The dried sludge is incinerated or chemically stabilized and disposed of as final waste (ash or residue).
Approximate inputs and energy required to treat aqueous sludge
Input power
| (data) item | Approximate quantity (kWh/kg sludge) |
| Electric power (for dewatering and pumps) | 0.2 to 0.5 kWh |
Input fuel
| type | Approximate quantity (kg) |
| Natural gas or fuel oil (for drying) | 0.05 to 0.1 kg |
Other than combustion, CO₂
| Approximate amount generated (kg CO₂/kg sludge) |
| 0.05 to 0.1 kg |
input
| name | Approximate input amount (kg/kg sludge) |
| Flocculant (e.g., polymer-based flocculant) | 0.01 to 0.02 kg |
| pH adjuster (e.g. sodium hydroxide, NaOH) | 0.01 to 0.03 kg |
| Water (for replenishment) | 0.1 to 0.2 kg |
Solvents and other process substances
| name | Approximate amount used (kg) | Cycloparametric rate |
| Cooling water (for heat exchange) | 2 to 4 kg | 90-95% (circulation used) |
| Solvent for cleaning (e.g., methanol, etc.) | 0.01 to 0.05 kg | 70-80% (reused) |
Waste requiring treatment
| name | Approximate quantity (kg/kg sludge) |
| Incinerator ash and sludge residue | 0.01 to 0.03 kg |
| Cleaning waste liquids (e.g., methanol waste) | 0.005 to 0.01 kg |
Process details and guidelines
- dehydration
- Aqueous sludge is dewatered to reduce the water content and concentrate the solids. Generally, polymer flocculants and pH adjusters (e.g., NaOH) are added, which flocculate the solids and facilitate filtration and centrifugal separation.
- Drying treatment
- The dewatered sludge is dried to further reduce its moisture content to facilitate incineration or final disposal. A heat source using natural gas or fuel oil is typically used, which evaporates the moisture.
- Incineration and solidification
- The dried sludge is either incinerated or stabilized with a solidifier (e.g., cement or lime) and managed as final waste. Ashes and residues generated in this process should be managed as incinerated ash or sludge residue.
summary
| (data) item | Approximate values |
| Power input (for dewatering and pumping) | 0.2 to 0.5 kWh |
| Input fuel (natural gas or fuel oil) | 0.05 to 0.1 kg |
| Other than combustion, CO₂ | 0.05 to 0.1 kg |
| Input Item Name | Approximate input amount (kg) |
| Coagulant (polymer-based) | 0.01 to 0.02 kg |
| pH adjuster (e.g., NaOH) | 0.01 to 0.03 kg |
| Water (for replenishment) | 0.1 to 0.2 kg |
| Solvents and other process substances Name | Approximate amount used (kg) | Cycloparametric rate |
| Cooling water (for heat exchange) | 2 to 4 kg | 90-95% of |
| Solvent for cleaning (e.g., methanol) | 0.01 to 0.05 kg | 70-80% of the total |
| Waste requiring treatment Name | Approximate quantity (kg) |
| Incinerator ash and sludge residue | 0.01 to 0.03 kg |
| Cleaning waste liquids (e.g., methanol waste) | 0.005 to 0.01 kg |
Organic liquid waste treatment
Incineration and oxidation** are commonly used to treat organic wastewater industrially. These methods use high temperatures to decompose organic components and safely dispose of hazardous materials. Below is a rough estimate of the input, energy consumption, and waste for treating 1 kg of organic liquid waste.
Treatment process for organic liquid waste
The main processing methods are as follows
- Incineration
- Organic wastewater is burned at high temperatures (800-1200°C) to decompose toxic components and ultimately to ash and gaseous products.
- Oxidation
- Oxidants (e.g., hydrogen peroxide or ozone) are used to break down organic components and convert them into harmless components.
Approximate input and energy requirements for organic liquid waste treatment
Input power
| (data) item | Approximate quantity (kWh/kg) |
| Power (for pumps and controls) | 0.3 to 0.5 kWh |
Input fuel
| type | Approximate quantity (kg) |
| Natural gas or fuel oil (for incineration) | 0.1 to 0.2 kg |
Other than combustion, CO₂
| Approximate amount generated (kg CO₂/kg organic liquid waste) |
| 0.1 to 0.15 kg |
input
| name | Approximate input amount (kg/kg effluent) |
| Oxidants (e.g., hydrogen peroxide, H₂O₂) | 0.05 to 0.1 kg |
| Auxiliary fuel (e.g., propane gas) | 0.02 to 0.05 kg |
| Cooling water (for temperature control) | 0.2 to 0.3 kg |
Solvents and other process substances
| name | Approximate amount used (kg) | Cycloparametric rate |
| Cooling water (for heat exchange) | 5-8 kg | 80-90% (circulation used) |
| Cleaning solvent (e.g., methanol) | 0.01 to 0.05 kg | 70-80% (reused) |
Waste requiring treatment
| name | Approximate quantity (kg/kg effluent) |
| Incinerator ash (may contain toxic substances) | 0.01 to 0.02 kg |
| Cleaning waste liquids (e.g., methanol waste) | 0.005 to 0.01 kg |
Process details and guidelines
- high-temperature incineration
- Organic liquid waste is burned at high temperatures (800-1200°C) to convert harmful organic components into harmless substances. This generates carbon dioxide (CO₂) as a byproduct.
- oxidation process
- An oxidizing agent such as hydrogen peroxide is added to decompose organic components in organic waste liquids through a chemical reaction, converting them into water and carbon dioxide.
- Cooling and separation process
- After incineration, the gas is cooled to separate the gas phase from the solid phase. Large volumes of water are used for cooling, and these are typically reused in a recirculating system.
- Cleaning and final waste management
- If incinerator ash or sludge are generated, they are disposed of properly and further stabilized if they contain harmful heavy metals or other contaminants.
ammonia
The Haber-Bosch process** is the main method used to produce ammonia industrially. In this process, nitrogen (N₂) and hydrogen (H₂)** react under high temperature and pressure to produce ammonia (NH₃). Since hydrogen is usually produced by steam reforming natural gas (methane), this process requires a large amount of energy and raw materials.
Approximate inputs and energy for producing 1 kg of ammonia
Input power
| (data) item | Approximate quantity (kWh/kg NH₃) |
| Electric power (for compression and pumps) | 0.6 to 1.0 kWh |
Input fuel
| type | Approximate quantity (kg) |
| Natural gas (methane, CH₄) | 0.5 to 0.6 kg |
Other than combustion, CO₂
| Approximate amount generated (kg CO₂/kg NH₃) |
| 2.8 to 3.0 kg |
input
| name | Approximate amount of input (kg/kg NH₃) |
| Nitrogen (N₂) | 0.8 to 0.9 kg |
| Hydrogen (H₂) | 0.2 to 0.3 kg |
Solvents and other process substances
| name | Approximate amount used (kg) | Cycloparametric rate |
| Cooling water (for reaction temperature control) | 5-10 kg | 85-95% (circulation use) |
| Lubricants (for equipment) | 0.01 to 0.02 kg | 90-95% (circulation used) |
Waste requiring treatment
| name | Approximate amount (kg/kg NH₃) |
| Unreacted gas and waste gas | 0.05 to 0.1 kg |
| Waste cooling water and sludge | 0.01 to 0.02 kg |
Manufacturing Process Overview
- steam reforming
- Steam reforming of natural gas (methane) to produce hydrogen and carbon monoxide (CO).
Reaction equation:
CH4+H2O→CO+3H2\text{CH}_4 + \text{H}_2\text{O} \rightarrow \text{CO} + 3\text{H}_2CH4+H2O→CO+3H2
- Aqueous gas shift reaction
- The carbon monoxide (CO) produced is further reacted with water vapor to produce additional hydrogen (H₂) and carbon dioxide (CO₂).
Reaction equation:
CO+H2O→CO2+H2\text{CO} + \text{H}_2\text{O} \rightarrow \text{CO}_2 + \text{H}_2CO+H2O→CO2+H2
- Nitrogen supply and reaction
- Nitrogen (N₂) obtained in the air separation process reacts with hydrogen (H₂) to produce ammonia.
Reaction equation:
N2+3H2→2NH3\text{N}_2 + 3\text{H}_2 \rightarrow 2\text{NH}_3N2+3H2→2NH3
This production process is energy-intensive, and carbon dioxide emissions, mainly from the reforming of natural gas, are a major environmental challenge. With the latest technologies and process improvements, attempts are being made to improve energy efficiency and reduce CO₂ emissions.
ion exchange resin
The main substances used in ion exchange resins and their share of the market are listed below:
- Polystyrene-Based Resins
- Market share: approx. 60
- Polystyrene-based resins are the primary raw material for ion exchange resins due to their high strength and durability and relatively low manufacturing costs. In particular, they are widely used in the manufacture of cation and anion exchange resins.
- Acrylic-Based Resins
- Market share: approx. 25
- Acrylic resins have become an important material because of their excellent chemical resistance and widespread use in certain applications (e.g., pharmaceuticals and food processing). Demand is growing, especially in niche markets such as high-purity water treatment
- Phenolic Resins
- Market share: approx. 15
- Phenolic resins are mainly used in special ion exchange applications that need to withstand high temperature environments and strong acid and alkali conditions, and are characterized by their durability and strength [1].
The total market share of these three types of resins is 100%, and the most suitable resin is selected according to the application.
activated carbon filter
Two main steps are involved in the production of activated carbon filters: carbonization of the raw material and activation process. This includes the process of treating the carbonized material at high temperatures and then activating it with oxidants or steam. Below is a summary of the inputs and approximate energy consumption for producing 1 kg of activated carbon filter.
- approximate inputs and energy for the activated carbon filter production process
Input power
| (data) item | Approximate quantity (kWh/kg activated carbon) |
| Power (for crushing, forming and compaction) | 0.4 to 0.6 kWh |
Input fuel
| type | Approximate quantity (kg) |
| Natural gas (for heating and activation) | 0.2 to 0.3 kg |
Other than combustion, CO₂
| Approximate amount generated (kg CO₂/kg activated carbon) |
| 0.1 to 0.15 kg |
input
| name | Approximate input amount (kg/kg activated carbon) |
| Raw materials (wood, coconut shells, coal, etc.) | 2.5 to 3.5 kg |
| Oxidants (e.g., carbon dioxide, CO₂) | 0.5 to 0.7 kg |
| Steam (for activation) | 0.3 to 0.5 kg |
Solvents and other process substances
| name | Approximate amount used (kg) | Cycloparametric rate |
| Cooling water (for temperature control) | 5-8 kg | 80-90% (circulation used) |
| Cleaning solvent (e.g., methanol) | 0.02 to 0.05 kg | 70-80% (reused) |
Waste requiring treatment
| name | Approximate quantity (kg/kg activated carbon) |
| Carbonization residue and unburned carbon | 0.02 to 0.05 kg |
| Cleaning waste liquids (e.g., methanol waste) | 0.01 to 0.02 kg |
- process details and description
- Raw material selection and carbonization:
- Raw materials such as wood, coconut shells, and coal are heated in a carbonization furnace to remove volatile components and convert them into carbon material. Natural gas or fuel oil is usually used as fuel during this carbonization stage.
- Activation process:
- The carbonized material is activated with an oxidant (e.g., carbon dioxide or steam) to form numerous pores inside. These pores determine the adsorption performance as activated carbon.
- Cooling and cleaning:
- After the activation process, the activated carbon is cooled and washed to remove residues. Cooling water is typically reused in a circulation system.
- Drying and finishing:
- Finally, the activated carbon is dried and processed as a final product in granular, powdered, or pellet form.
Considerations in Manufacturing Methods
Depending on the raw material used for production (wood, coconut shells, or coal), inputs and energy consumption vary slightly. In particular, coconut shells, while more expensive, have superior adsorption performance and are highly valued in air purification applications.
sulfuric acid
The most common industrial production of sulfuric acid (H₂SO₄) is by the Contact Process. In this process, sulfur dioxide (SO₂) is produced primarily by oxidizing sulfur (S), which is then oxidized to sulfur trioxide (SO₃), and finally water is added to produce sulfuric acid.
Approximate input and energy required to produce 1 kg of sulfuric acid
Input power
| (data) item | Approximate quantity (kWh/kg) |
| Electric power (for pumps and compressors) | 0.2 to 0.3 kWh |
Input fuel
| type | Approximate quantity (kg) |
| Natural gas or fuel oil | 0.1 to 0.15 kg |
Other than combustion, CO₂
| Approximate amount generated (kg CO₂/kg H₂SO₄) |
| 0.01 to 0.02 kg |
input
| name | Approximate amount of input (kg/kg H₂SO₄) |
| Sulfur (S) | 0.32 to 0.35 kg |
| Oxygen (O₂, from air) | 0.15 to 0.2 kg |
| Water (H₂O) | 0.1 to 0.15 kg |
Solvents and other process substances
| name | Approximate amount used (kg) | Cycloparametric rate |
| Cooling water (for temperature control) | 5-8 kg | 85-95% (circulation use) |
| Lubricants (for equipment) | 0.01 to 0.02 kg | 90-95% (circulation used) |
Waste requiring treatment
| name | Approximate amount (kg/kg H₂SO₄) |
| Waste catalyst (e.g., vanadium oxide) | 0.001 to 0.003 kg |
| Waste washing liquid (water containing sulfuric acid) | 0.01 to 0.02 kg |
Manufacturing Process Overview
- Oxidation of sulfur: Sulfur is burned to produce SO₂.
- Oxidation of SO₂ : Oxidized to SO₃ using vanadium catalyst.
- SO₃ absorption: Process in which SO₃ is absorbed by water to produce sulfuric acid.
caustic soda
The **Chlor-Alkali Process** is primarily used to produce caustic soda (NaOH) industrially. This process electrolyzes a sodium chloride (NaCl) solution to produce caustic soda, chlorine (Cl₂), and hydrogen (H₂). Below are the approximate inputs, energy consumption, and waste for producing 1 kg of caustic soda.
Approximate Inputs and Energy Consumption in Caustic Soda Production Process
Input power
| (data) item | Approximate quantity (kWh/kg NaOH) |
| Electric power (for electrolysis) | 2.2 to 2.7 kWh |
Input fuel
| type | Approximate quantity (kg) |
| Natural gas (for heating) | 0.1 to 0.15 kg |
Other than combustion, CO₂
| Approximate amount generated (kg CO₂/kg NaOH) |
| 0 kg |
input
| name | Approximate input amount (kg/kg NaOH) |
| Sodium chloride (NaCl) | 1.5 to 1.8 kg |
| Water (H₂O) | 0.5 to 0.8 kg |
Solvents and other process substances
| name | Approximate amount used (kg) | Cycloparametric rate |
| Cooling water (for temperature control) | 8-12 kg | 90-95% (circulation used) |
| Lubricating oil (for equipment) | 0.01 to 0.02 kg | 90-95% (circulation used) |
Waste requiring treatment
| name | Approximate amount (kg/kg NaOH) |
| Waste brine (containing unreacted NaCl) | 0.1 to 0.2 kg |
| Chlorine gas by-product (Cl₂) | 0.4-0.5 kg (reusable) |
Manufacturing Process Details
- Electrolysis of salt water:
- Saturated brine (sodium chloride solution) is fed into the electrolyzer to produce chlorine (Cl₂) at the anode and hydrogen (H₂) and caustic soda at the cathode [163].
Reaction equation:
2NaCl+2H2O→Cl2+H2+2NaOH2\text{NaCl} + 2\text{H}_2\text{O} \rightarrow \text{Cl}_2 + \text{H}_2 + 2\text{NaOH}2NaCl+2H2O→ Cl2+H2+2NaOH
- Separation of chlorine gas and hydrogen gas:
- The chlorine and hydrogen produced in the electrolyzer are separated and the chlorine is used in the chemical industry to produce other compounds (e.g., production of vinyl chloride).
- Concentration and purification of caustic soda:
- Caustic soda obtained by electrolysis is typically 10-12% in concentration and is concentrated to 50% in an evaporative concentrator. This concentration process requires heating with steam or natural gas.
- Reuse of waste brine:
- After electrolysis, unreacted sodium chloride is concentrated again and returned to the electrolyzer, increasing the overall process feedstock efficiency.
Waste activated carbon treatment
Two methods are often used to treat waste activated carbon: reactivation (regeneration) processes and incineration. The reactivation process involves thermal or chemical treatment to remove adsorbed contaminants so that the activated carbon can be reused. Incineration, on the other hand, is the process of choice, especially when the contaminants are highly hazardous. Below are the inputs, energy consumption, and waste estimates for processing 1 kg of waste activated carbon.
Approximate input and energy consumption for processing 1 kg of waste activated carbon
Input power
| (data) item | Approximate quantity (kWh/kg) |
| Electric power (for heating and pumps) | 0.5 to 1.0 kWh |
Input fuel
| type | Approximate quantity (kg) |
| Natural gas or fuel oil (for heating) | 0.2 to 0.3 kg |
Other than combustion, CO₂
| Approximate amount generated (kg CO₂/kg waste activated carbon) |
| 0.1 to 0.15 kg |
input
| name | Approximate input amount (kg/kg waste activated carbon) |
| Oxidants (e.g., carbon dioxide, CO₂) | 0.3 to 0.5 kg |
| Steam (for activation) | 0.2 to 0.3 kg |
Solvents and other process substances
| name | Approximate amount used (kg) | Cycloparametric rate |
| Cooling water (for heat exchange) | 5-8 kg | 80-90% (circulation used) |
| Cleaning solvent (e.g., methanol) | 0.01 to 0.05 kg | 70-80% (reused) |
Waste requiring treatment
| name | Approximate quantity (kg/kg waste activated carbon) |
| Incinerator ash or carbonization residue | 0.02 to 0.05 kg |
| Cleaning waste liquids (e.g., methanol waste) | 0.01 to 0.02 kg |
Process Details
- Reactivation Process:
During reactivation, thermal regeneration (approx. 800-900°C) is used to thermally decompose adsorbed organic compounds and contaminants. Steam and oxidants (CO₂ or air) are used in this process to reshape the internal structure of the waste activated carbon and restore its adsorption capacity. -
Incineration:
In incineration, waste activated carbon is burned at high temperatures for complete decomposition, especially if it contains toxic substances. As byproducts, carbon dioxide (CO₂) and other oxidation products are generated, leaving ash as waste. - Cooling and Cleaning:
After reactivation or incineration of activated carbon, a cooling process is required. Cooling water usage is high, but is typically circulated.
waste acid treatment
Methods such as neutralization and membrane separation are often used to treat 1 kg of waste acid (especially acidic waste liquids such as hydrochloric acid and sulfuric acid). Below is a rough estimate of inputs and energy consumption for common treatment methods.
Approximate input and energy required to process 1 kg of waste acid
Input power
| (data) item | Approximate quantity (kWh/kg) |
| Power (for pumps and membrane separation) | 0.3 to 0.5 kWh |
Input fuel
| type | Approximate quantity (kg) |
| Natural gas or fuel oil | 0.05 to 0.1 kg |
Other than combustion, CO₂
| Approximate amount generated (kg CO₂/kg waste acid) |
| 0.05 to 0.1 kg |
input
| name | Approximate input amount (kg/kg waste acid) |
| Neutralizer (e.g., limestone, CaCO₃) | 0.1 to 0.2 kg |
| Oxidants (e.g., hydrogen peroxide) | 0.05 to 0.1 kg |
| Water (H₂O, for dilution) | 0.2 to 0.4 kg |
Solvents and other process substances
| name | Approximate amount used (kg) | Cycloparametric rate |
| Cooling water (for heat exchange) | 4-6 kg | 85-90% (circulation used) |
| Cleaning solvent (e.g., methanol) | 0.02 to 0.05 kg | 70-80% (reused) |
Waste requiring treatment
| name | Approximate quantity (kg/kg waste acid) |
| Neutralization product (salt sludge) | 0.05 to 0.1 kg |
| Cleaning waste liquids (e.g., methanol waste) | 0.01 to 0.02 kg |
Process Details
- neutralizing treatment
- Waste acid is treated with a neutralizing agent (e.g., limestone or sodium hydroxide) and disposed of as harmless salts. This neutralization reaction generates heat, which requires a large amount of cooling water for cooling.
- Membrane separation process
- Diffusion dialysis or membrane distillation is sometimes used to recover metal components from waste acid. In this method, the acid is regenerated and the metal components can be recovered separately.
- Final Waste Management
- Sludges and salts produced after treatment must be managed as solid waste. Depending on the type of byproducts produced in the neutralization reaction, further stabilization or incineration treatment may be required.
Waste Alkali Treatment
The neutralization treatment method is generally used to treat 1 kg of waste alkali, using an acid to neutralize the reaction. The following is a rough estimate of the input and energy consumption for this treatment.
Treatment process for 1 kg of waste alkali
Input power
- 0.2~0.4 kWh/kg
Input fuel
- Natural gas or fuel oil: 0.05 to 0.1 kg
Other than combustion, CO₂
- 0.05 to 0.1 kg
input
- Acid (e.g., sulfuric acid): 0.1-0.15 kg
- Water (for dilution): 0.2-0.4 kg
Solvents and other process substances
- Cooling water: 5-7 kg (circulation rate: 90-95%)
Waste requiring treatment
- Neutralizing sludge: 0.05-0.1 kg
phosphoric acid (H3PO4)
Phosphoric acid (H₃PO₄) is produced by two main methods: **Wet Process** and Thermal Process**. The wet process is the most common and involves the reaction of phosphate ore with sulfuric acid. This process efficiently produces phosphoric acid, which is widely used in fertilizer and industrial applications.
Approximate input and energy for producing 1 kg of phosphoric acid
Input power
| (data) item | Approximate quantity (kWh/kg H₃PO₄) |
| Electric power (for grinding, pumps and filtration) | 0.1 to 0.2 kWh |
Input fuel
| type | Approximate quantity (kg) |
| Natural gas or fuel oil (for heating) | 0.05 to 0.1 kg |
Other than combustion, CO₂
| Approximate amount generated (kg CO₂/kg H₃PO₄) |
| 0.02 to 0.05 kg |
input
| name | Approximate input amount (kg/kg H₃PO₄) |
| Phosphorus ore (Ca₃(PO₄)₂) | 1.5 to 2.0 kg |
| Sulfuric acid (H₂SO₄, 98% concentration) | 0.5 to 0.6 kg |
| Water (H₂O, for dilution) | 0.3 to 0.5 kg |
Solvents and other process substances
| name | Approximate amount used (kg) | Cycloparametric rate |
| Cooling water (for temperature control) | 4-6 kg | 85-90% (circulation used) |
| Lubricants (for equipment) | 0.01 to 0.02 kg | 90-95% (circulation used) |
Waste requiring treatment
| name | Approximate amount (kg/kg H₃PO₄) |
| By-product (phosphogypsum, CaSO₄-2H₂O) | 2.5 to 3.0 kg |
| Cleaning waste liquid (acidic waste liquid) | 0.1 to 0.2 kg |
Process Details
- Phosphate ore crushing
- Calcium phosphate (Ca₃(PO₄)₂) is ground to improve reactivity.
- Reaction with sulfuric acid
- Phosphate and phosphogypsum (CaSO₄-2H₂O) are produced by the reaction of phosphate ore with sulfuric acid (H₂SO₄).
- Filtration and Separation
- The phosphogypsum produced is filtered to separate the phosphoric acid. Phosphogypsum is produced in large quantities as a byproduct and must be properly treated or reused.
zinc sulfate (ZnSO4) (sulphate)
Zinc sulfate (ZnSO₄) is typically produced by reacting zinc metal or zinc ore with sulfuric acid. Below are the approximate inputs, energy consumption, and waste for producing 1 kg of zinc sulfate.
Approximate Inputs and Energy for Zinc Sulfate Production Process
Input power
| (data) item | Approximate quantity (kWh/kg ZnSO₄) |
| Electric power (for grinding, dissolution and filtration) | 0.1 to 0.2 kWh |
Input fuel
| type | Approximate quantity (kg) |
| Natural gas or fuel oil (for heating) | 0.05 to 0.1 kg |
Other than combustion, CO₂
| Approximate amount generated (kg CO₂/kg ZnSO₄) |
| 0.01 to 0.03 kg |
input
| name | Approximate input amount (kg/kg ZnSO₄) |
| Zinc (Zn, metal or ore) | 0.3 to 0.4 kg |
| Sulfuric acid (H₂SO₄, 98% concentration) | 0.5 to 0.6 kg |
| Water (H₂O, for dilution) | 0.2 to 0.3 kg |
Solvents and other process substances
| name | Approximate amount used (kg) | Cycloparametric rate |
| Cooling water (for temperature control) | 3-5 kg | 85-95% (circulation use) |
| Lubricants (for equipment) | 0.01 to 0.02 kg | 90-95% (circulation used) |
Waste requiring treatment
| name | Approximate amount (kg/kg ZnSO₄) |
| Sludge containing impurities (Fe, Pb, Al, etc.) | 0.02 to 0.05 kg |
| Cleaning waste liquid (acidic waste liquid) | 0.01 to 0.02 kg |
Manufacturing Process Details
- Zinc Dissolution: Dissolving zinc metal or zinc ore (such as zinc oxide or sulfide ore) in sulfuric acid (H₂SO₄) produces zinc sulfate. Hydrogen gas (H₂) is produced as a byproduct of this reaction.
- Filtration and purification: After dissolution, the product is filtered to remove impurities to obtain a zinc sulfate solution. If necessary, water is added to adjust the concentration.
- Crystallization and drying: The zinc sulfate solution is cooled and crystallized to obtain solid zinc sulfate. The crystals are then dried and adjusted to the desired particle size for commercialization.
sulfur dioxide
The industrial production of sulfur dioxide (SO₂) is primarily done through the combustion of sulfur. In this process, sulfur is reacted with oxygen to produce sulfur dioxide, which is used, for example, in the production of sulfuric acid.
Approximate input and energy for producing 1 kg of sulfur dioxide
Input power
| (data) item | Approximate quantity (kWh/kg SO₂) |
| Electric power (for airflow and compression) | 0.2 to 0.4 kWh |
Input fuel
| type | Approximate quantity (kg) |
| Natural gas or fuel oil (for heating) | 0.05 to 0.1 kg |
Other than combustion, CO₂
| Approximate amount generated (kg CO₂/kg SO₂) |
| 0.01 to 0.02 kg |
input
| name | Approximate amount of input (kg/kg SO₂) |
| Sulfur (S) | 0.5 to 0.6 kg |
| Oxygen (O₂, from air) | 0.2 to 0.3 kg |
Solvents and other process substances
| name | Approximate amount used (kg) | Cycloparametric rate |
| Cooling water (for temperature control) | 4-6 kg | 85-95% (circulation use) |
| Lubricating oil (for equipment) | 0.01 to 0.02 kg | 90-95% (circulation used) |
Waste requiring treatment
| name | Approximate amount (kg/kg SO₂) |
| Unreacted sulfur and sludge | 0.01 to 0.02 kg |
| Cleaning waste liquid (acidic waste liquid) | 0.005 to 0.01 kg |
Process Details
- Combustion of sulfur
- Pure sulfur is burned and reacts with oxygen to produce sulfur dioxide. Combustion typically takes place at temperatures of 700-900°C, and the sulfur dioxide produced is recovered as a gas [206].
- Cooling and refining
- The sulfur dioxide gas produced is cooled to remove residues. Cooling water is typically used in this process and reused in the circulation system.
- Control of byproducts
- The sulfur combustion process can leave behind unreacted sulfur, which is filtered out and either reused or discarded.
Water-based drilling fluid
Water-Based Drilling Fluid is a fluid used when drilling oil and gas wells, primarily for well cooling, lubrication, and borehole wall stabilization. Below are the inputs and approximate energy consumption for producing 1 kg of water-based drilling fluid.
Approximate inputs and energy for producing 1 kg of aqueous drilling fluid
Input power
| (data) item | Approximate quantity (kWh/kg drilling fluid) |
| Electric power (for stirring and mixing) | 0.1 to 0.3 kWh |
Input fuel
| type | Approximate quantity (kg) |
| Natural gas or fuel oil (for heating) | 0.05 to 0.1 kg |
Other than combustion, CO₂
| Approximate amount generated (kg CO₂/kg drilling fluid) |
| 0.02 to 0.05 kg |
input
| name | Approximate amount of input (kg/kg drilling fluid) |
| Bentonite (clay) | 0.05 to 0.1 kg |
| Sodium chloride (NaCl) | 0.03 to 0.05 kg |
| Polymers (lubricants, viscosity regulators, etc.) | 0.01 to 0.02 kg |
| Water (H₂O, base fluid) | 0.8 to 0.9 kg |
Solvents and other process substances
| name | Approximate amount used (kg) | Cycloparametric rate |
| Cooling water (for heat exchange) | 5 to 7 kg | 85-90% (circulation used) |
| Lubricants (for equipment) | 0.01 to 0.02 kg | 90-95% (circulation used) |
Waste requiring treatment
| name | Approximate quantity (kg/kg drilling fluid) |
| Solid residues (e.g., well cuttings) | 0.01 to 0.03 kg |
| Wastewater (organic matter contained, clay, etc.) | 0.02 to 0.04 kg |
Process Details and Descriptions
- Stirring and mixing:
- Base water and various additives (bentonite, sodium chloride, polymers, etc.) are mixed to create a homogeneous fluid. The ratio of these components is important to adjust viscosity and ensure stability in the well
- Cooling and temperature control:
- Cooling water is used to maintain fluid properties under high temperature conditions. It is usually used in a circulating system, which means that even though the amount of cooling water used is high, the circulation rate is high.
- Waste disposal and management:
- After use, the drilling fluid contains cuttings (rock fragments from drilling) generated during well drilling, which must be properly separated and treated. The organic matter, clay, and other wastes it contains are also managed in accordance with environmental regulations.
Oil-based drilling fluid
Oil-Based Mud (OBM) is an important fluid used primarily when drilling oil and gas wells to lubricate and cool the wellbore and maintain well wall stability. Below is a rough estimate of the inputs, energy consumption, and waste to produce 1 kg of oil-based drilling fluid.
Approximate input and energy for producing 1 kg of oil-based drilling fluid
Input power
| (data) item | Approximate quantity (kWh/kg OBM) |
| Electric power (for stirring and mixing) | 0.2 to 0.5 kWh |
Input fuel
| type | Approximate quantity (kg) |
| Natural gas or fuel oil (for heating) | 0.1 to 0.2 kg |
Other than combustion, CO₂
| Approximate amount generated (kg CO₂/kg OBM) |
| 0.05 to 0.1 kg |
input
| name | Approximate amount of input (kg/kg OBM) |
| Base oil (mineral or synthetic) | 0.6-0.7 kg |
| Emulsifier (surfactant) | 0.05 to 0.1 kg |
| Viscosity regulator (e.g., bentonite) | 0.02 to 0.05 kg |
| Weight regulator (e.g., barite) | 0.1 to 0.3 kg |
| Water (H₂O, for emulsion formation) | 0.1 to 0.15 kg |
Solvents and other process substances
| name | Approximate amount used (kg) | Cycloparametric rate |
| Cooling water (for temperature control) | 4-6 kg | 90-95% (circulation used) |
| Lubricating oil (for equipment) | 0.01 to 0.02 kg | 90-95% (circulation used) |
Waste requiring treatment
| name | Approximate quantity (kg/kg OBM) |
| Solid residues (e.g., well cuttings) | 0.05 to 0.1 kg |
| Waste oil-based mud (containing oil, clay, etc.) | 0.02 to 0.05 kg |
Process Details and Descriptions
- Base oil selection and mixing:
Base oil (mineral or synthetic) is uniformly mixed with various additives (emulsifiers, viscosity regulators, weight regulators, etc.) to form an oily drilling fluid. This improves lubrication performance and temperature stability during drilling. - Cooling and Temperature Control:
Cooling water is used to maintain flow characteristics in high temperature environments. Cooling systems are typically circulated, so even though the amount of cooling water used is high, the environmental impact is low. - Waste Management
: After use, drilling fluids contain cuttings and waste oily mud from well drilling, which must be properly separated and treated. In particular, oily wastes must be managed according to strict environmental standards.
Synthetic base drilling fluid
Synthetic-Based Drilling Fluid (SBF) is a drilling fluid used under special conditions, especially in areas with strict environmental regulations or in deep drilling. Typically, synthetic oils (e.g., polyolefins, esters, polyalphaolefins) are used as base oils to provide stable physical properties and excellent lubrication characteristics. Below are the inputs and approximate energy consumption for producing 1 kg of synthetic base drilling fluid.
Approximate input and energy for producing 1 kg of synthetic base drilling fluid
Input power
| (data) item | Approximate quantity (kWh/kg SBF) |
| Electric power (for stirring and mixing) | 0.3 to 0.5 kWh |
Input fuel
| type | Approximate quantity (kg) |
| Natural gas or fuel oil (for heating) | 0.1 to 0.15 kg |
Other than combustion, CO₂
| Approximate amount generated (kg CO₂/kg SBF) |
| 0.05 to 0.1 kg |
input
| name | Approximate amount of input (kg/kg SBF) |
| Synthetic base oils (polyolefins, esters, PAOs, etc.) | 0.7 to 0.8 kg |
| Emulsifier (surfactant) | 0.05 to 0.1 kg |
| Viscosity regulator (e.g., bentonite) | 0.02 to 0.04 kg |
| Weight regulator (e.g., barite) | 0.1 to 0.2 kg |
| Water (H₂O, for emulsion formation) | 0.05 to 0.1 kg |
Solvents and other process substances
| name | Approximate amount used (kg) | Cycloparametric rate |
| Cooling water (for temperature control) | 3-5 kg | 85-90% (circulation used) |
| Lubricants (for equipment) | 0.01 to 0.02 kg | 90-95% (circulation used) |
Waste requiring treatment
| name | Approximate quantity (kg/kg SBF) |
| Solid residues (e.g., well cuttings) | 0.05 to 0.1 kg |
| Waste drilling fluid (containing synthetic oil, clay, etc.) | 0.02 to 0.05 kg |
Process Details and Considerations
- Base oil mixing and emulsification
Synthetic base oil is uniformly mixed with various additives (emulsifiers, viscosity adjusters, weight adjusters, etc.) to form synthetic base drilling fluid. This improves lubrication performance and temperature stability during drilling. - Cooling and Temperature
Control To maintain flow characteristics under high temperature conditions, cooling water is used to control temperature. Cooling water is often circulated and has a low environmental impact even though the amount used is high. - Waste Management
After use, drilling fluids contain waste mud containing cuttings and synthetic oil produced during well drilling, which must be properly separated and treated.
aluminum sulfate (AlSO4)
Aluminum sulfate (aluminum sulfate, Al₂(SO₄)₃) is commonly produced primarily from aluminum hydroxide (Al(OH)₃) or bauxite, using **sulfuric acid (H₂SO₄)**. This process can be done both wet and dry, and the product can be in liquid or solid form. Below are approximate inputs, energy consumption, and waste for the production of 1 kg of aluminum sulfate.
Approximate input and energy consumption for producing 1 kg of aluminum sulfate
Input power
| (data) item | Approximate amount (kWh/kg Al₂(SO₄)₃) |
| Electric power (for stirring and mixing) | 0.2 to 0.3 kWh |
Input fuel
| type | Approximate quantity (kg) |
| Natural gas or fuel oil (for heating) | 0.05 to 0.1 kg |
Other than combustion, CO₂
| Approximate amount generated (kg CO₂/kg Al₂(SO₄)₃) |
| 0.01 to 0.02 kg |
input
| name | Approximate input amount (kg/kg Al₂(SO₄)₃) |
| Aluminum hydroxide (Al(OH)₃) | 0.3 to 0.4 kg |
| Sulfuric acid (H₂SO₄, 98% concentration) | 0.4 to 0.5 kg |
| Water (H₂O, for dilution) | 0.2 to 0.3 kg |
Solvents and other process substances
| name | Approximate amount used (kg) | Cycloparametric rate |
| Cooling water (for temperature control) | 4-6 kg | 85-90% (circulation used) |
| Lubricants (for equipment) | 0.01 to 0.02 kg | 90-95% (circulation used) |
Waste requiring treatment
| name | Approximate amount (kg/kg Al₂(SO₄)₃) |
| Unreacted sludge (containing impurities) | 0.02 to 0.04 kg |
| Cleaning waste liquid (acidic waste liquid) | 0.01 to 0.02 kg |
Process Overview and Description
- Dissolution of aluminum hydroxide:
Aluminum hydroxide reacts with sulfuric acid to produce aluminum sulfate. This reaction is exothermic and usually proceeds by raising the temperature to about 170°C. The reaction takes 10 to 12 minutes to complete, and the pressure reaches
about 5 to 6 bar.
- Filtration and Purification:
The reaction product is cooled and filtered to remove dissolved residues and impurities. It is
then separated as a liquid or crystallized product
The following is a list of the most common problems with the
- Crystallization and Drying:
Liquid aluminum sulfate is concentrated and crystallized using an evaporator. During this crystallization process, the crystals are cooled and packed as the final product.
Poly DADMAC
Poly DADMAC (polydaryldimethylammonium chloride) is a cationic polymer produced primarily by radical polymerization. The production process uses **DADMAC (diallyl dimethyl ammonium chloride)** as the main monomer, and the product is obtained through a chemical reaction and purification process. Below are the approximate inputs, energy consumption, and waste for the production of 1 kg of poly DADMAC.
Approximate inputs and energy for producing 1 kg of poly DADMAC
Input power
| (data) item | Approximate quantity (kWh/kg Poly DADMAC) |
| Electric power (for stirring and mixing) | 0.5 to 0.8 kWh |
Input fuel
| type | Approximate quantity (kg) |
| Natural gas or fuel oil (for heating) | 0.1 to 0.15 kg |
Other than combustion, CO₂
| Approximate amount generated (kg CO₂/kg Poly DADMAC) |
| 0.03 to 0.05 kg |
input
| name | Approximate input (kg/kg Poly DADMAC) |
| DADMAC (monomer) | 0.6 to 0.8 kg |
| Initiator (e.g., ammonium persulfate) | 0.02 to 0.05 kg |
| Water (H₂O, for solvent and dilution) | 0.4 to 0.6 kg |
Solvents and other process substances
| name | Approximate amount used (kg) | Cycloparametric rate |
| Cooling water (for heat exchange) | 5-8 kg | 85-95% (circulation use) |
| Cleaning solvent (e.g., ethanol) | 0.01 to 0.03 kg | 70-80% (reused) |
Waste requiring treatment
| name | Approximate quantity (kg/kg Poly DADMAC) |
| Sludge containing by-products and impurities | 0.02 to 0.05 kg |
| Cleaning waste liquid (solvent-containing waste liquid) | 0.01 to 0.02 kg |
Manufacturing Process Details
- Radical Polymerization Reaction:
Radical polymerization is carried out using daryldimethylammonium chloride (DADMAC) as a monomer and initiators such as ammonium persulfate. This polymerization reaction produces long chain polymers (poly-DADMAC). - Solvent and impurity removal: The
resulting polymer undergoes a washing process to remove impurities. Cooling water and cleaning solvents such as ethanol are used to purify the product. - Concentration and Drying:
The final product is concentrated and processed into the appropriate form (liquid or powder) for shipment as a product. A crystallization or drying process is often required.
Manufacturing Considerations
Temperature control is important for poly-DADMAC because the synthesis process involves an exothermic reaction. In addition, the effluents and sludges produced during production must be properly managed. These wastes must ultimately be disposed of or recycled in accordance with specific environmental regulations.
Iron(III) chloride
Ferric chloride (iron(III) chloride, FeCl₃) is produced industrially mainly by the reaction of **iron metal with dry chlorine (Cl₂)**. This method involves the reaction of iron with dry chlorine gas to produce ferric chloride under high temperatures. Below are the approximate inputs, energy consumption, and waste for the production of 1 kg of ferric chloride.
Approximate inputs and energy for producing 1 kg of ferric chloride
Input power
| (data) item | Approximate quantity (kWh/kg FeCl₃) |
| Power (agitation, compression and reaction applications) | 0.3 to 0.5 kWh |
Input fuel
| type | Approximate quantity (kg) |
| Natural gas or fuel oil (for heating) | 0.1 to 0.15 kg |
Other than combustion, CO₂
| Approximate amount generated (kg CO₂/kg FeCl₃) |
| 0.01 to 0.03 kg |
input
| name | Approximate input amount (kg/kg FeCl₃) |
| Iron (Fe, metal) | 0.3 to 0.4 kg |
| Chlorine (Cl₂, dry) | 0.5 to 0.6 kg |
| Water (H₂O, for dilution) | 0.1 to 0.15 kg |
Solvents and other process substances
| name | Approximate amount used (kg) | Cycloparametric rate |
| Cooling water (for heat exchange) | 4-6 kg | 85-95% (circulation use) |
| Lubricating oil (for equipment) | 0.01 to 0.02 kg | 90-95% (circulation used) |
Waste requiring treatment
| name | Approximate amount (kg/kg FeCl₃) |
| Sludge containing unreacted iron and impurities | 0.02 to 0.05 kg |
| Cleaning waste liquid (acidic waste liquid) | 0.01 to 0.02 kg |
Process Details and Considerations
- Iron-Chlorine Reaction:
Iron metal reacts with dry chlorine gas at high temperatures to form iron(III) chloride (FeCl₃). Typically, the reaction takes place at temperatures of 300-400°C and the chlorine gas must be supplied dry. - Product cooling and separation:
The generated ferric chloride gas is cooled and converted to liquid or solid form. In this process, cooling water is typically used in a circulation system to reduce environmental impact. - Waste Management: Proper waste management is required because sludges containing unreacted iron and impurities are generated as by-products. These wastes must be treated or reused in accordance with environmental regulations.
sodium carbonate (Na2CO3)
Sodium carbonate (Na₂CO₃) is mainly produced industrially by the **Solvay Process**. This process uses sodium chloride (NaCl) and limestone (CaCO₃) as raw materials and adds ammonia (NH₃) as a reaction mediator. Below are the approximate inputs, energy consumption, and waste for the production of 1 kg of sodium carbonate.
Approximate input and energy for producing 1 kg of sodium carbonate
Input power
| (data) item | Approximate quantity (kWh/kg Na₂CO₃) |
| Electric power (for stirring and mixing) | 0.2 to 0.3 kWh |
Input fuel
| type | Approximate quantity (kg) |
| Natural gas or fuel oil (for heating) | 0.15 to 0.25 kg |
Other than combustion, CO₂
| Approximate amount generated (kg CO₂/kg Na₂CO₃) |
| 0.05 to 0.1 kg |
input
| name | Approximate input amount (kg/kg Na₂CO₃) |
| Sodium chloride (NaCl) | 1.6 to 1.8 kg |
| Limestone (CaCO₃) | 1.2 to 1.4 kg |
| Ammonia (NH₃) | 0.1 to 0.15 kg (reused) |
| Water (H₂O, for dilution and cleaning) | 1.0 to 1.2 kg |
Solvents and other process substances
| name | Approximate amount used (kg) | Cycloparametric rate |
| Cooling water (for heat exchange) | 5-8 kg | 85-95% (circulation use) |
| Solvent for cleaning (e.g., water) | 0.5 to 1.0 kg | 70-80% (reused) |
Waste requiring treatment
| name | Approximate amount (kg/kg Na₂CO₃) |
| Calcium chloride (CaCl₂) produced as a by-product | 1.0 to 1.3 kg |
| Other effluents and residues | 0.1 to 0.2 kg |
Process Details and Environmental Impact
- Reaction Process:
In the Solvay process, limestone is first pyrolyzed to produce carbon dioxide (CO₂) and calcium oxide (CaO), and CaO is reacted with water to obtain calcium hydroxide (Ca(OH)₂). Sodium hydrogen carbonate (NaHCO₃) is then precipitated by passing ammonia and carbon dioxide through a sodium chloride solution, which is
finally heated (160-230°C) to produce sodium carbonate, water, and CO₂.
The following is a list of the most common problems with the “C” in the “C” column.
- Energy Efficiency:
The Solvay process is energy intensive because it involves both the decomposition of calcium at high temperatures (950-1100°C) and the pyrolysis of sodium bicarbonate. It also produces calcium salt waste, making
waste management a challenge.
The following is a list of the most common problems with the “C” in the “C” column.
- Waste and Byproduct Management:
The main waste product of the Solvay process is calcium chloride (CaCl₂), which is sometimes used as a snow-melting agent on roads or for industrial applications, but it is
difficult to dispose of all of it and can cause environmental contamination
ethylene
The industrial production of ethylene (C₂H₄) primarily uses the **steam cracking** method. Steam cracking involves heating hydrocarbons such as naphtha and ethane to very high temperatures (750-950°C) to produce smaller molecules (ethylene, propylene, etc.) through a series of thermal cracking reactions. Below are approximate inputs, energy consumption, and waste for the production of 1 kg of ethylene.
Approximate input and energy for producing 1 kg of ethylene
Input power
| (data) item | Approximate quantity (kWh/kg C₂H₄) |
| Power (for compression, mixing and separation) | 0.8 to 1.2 kWh |
Input fuel
| type | Approximate quantity (kg) |
| Natural gas or fuel oil (for heating) | 0.25 to 0.4 kg |
Other than combustion, CO₂
| Approximate amount generated (kg CO₂/kg C₂H₄) |
| 1.3 to 1.5 kg |
input
| name | Approximate amount of input (kg/kg C₂H₄) |
| Ethane (or naphtha) | 1.2 to 1.4 kg |
| Steam (for cracking) | 0.5 to 0.7 kg |
| Water (for cooling and separation) | 1.5 to 2.0 kg |
Solvents and other process substances
| name | Approximate amount used (kg) | Cycloparametric rate |
| Cooling water (for heat exchange and condensation) | 4-6 kg | 85-95% (circulation use) |
| Lubricating oil (for equipment) | 0.01 to 0.02 kg | 90-95% (circulation used) |
Waste requiring treatment
| name | Approximate amount (kg/kg C₂H₄) |
| Coke residue (catalyst surface deposits) | 0.05 to 0.1 kg |
| Unreacted hydrocarbons (waste gas) | 0.02 to 0.04 kg |
Process Details and Considerations
- Steam cracking process:
Ethane or naphtha is heated to very high temperatures and cooled rapidly to crack hydrocarbon molecules. It primarily produces lightweight olefins such as ethylene, propylene, and butadiene, but coke deposits can be a problem. - Cooling and Separation Process:
The gas produced is cooled to separate ethylene, propylene, and other by-products using different boiling points. This process requires the use of large volumes of cooling water and compressors. - Waste and Byproduct Management:
Steam cracking produces coke deposits and unreacted hydrocarbons as byproducts, requiring regular catalyst regeneration and waste gas management.
oxygen
Oxygen (O₂) is mainly produced using **Air Separation. This primarily employs Cryogenic Distillation (Cryogenic Distillation) and Pressure Swing Adsorption (Pressure Swing Adsorption, PSA)** methods, with Cryogenic Distillation commonly used to obtain high purity oxygen. In this process, air is cooled and liquefied, and oxygen is separated using different boiling points.
Below are the approximate inputs, energy consumption, and waste for producing 1 kg of oxygen.
Approximate input and energy required to produce 1 kg of oxygen
Input power
| (data) item | Approximate quantity (kWh/kg O₂) |
| Electric power (for compression and cooling) | 0.8 to 1.0 kWh |
Input fuel
| type | Approximate quantity (kg) |
| Fuel (natural gas for heating) | 0.05 to 0.1 kg |
Other than combustion, CO₂
| Approximate amount generated (kg CO₂/kg O₂) |
| 0.01 to 0.02 kg |
input
| name | Approximate amount of input (kg/kg O₂) |
| Air (raw material) | 4.5 to 5.5 kg |
| Cooling water (for use in the cooling process) | 1.0 to 1.5 kg |
Solvents and other process substances
| name | Approximate amount used (kg) | Cycloparametric rate |
| Cooling water (for heat exchange) | 5-8 kg | 85-95% (circulation use) |
| Lubricants (for equipment) | 0.01 to 0.02 kg | 90-95% (circulation used) |
Waste requiring treatment
| name | Approximate amount (kg/kg O₂) |
| Residual nitrogen and trace impurities | 0.02 to 0.05 kg |
Process Details and Considerations
- Cryogenic Distillation:
Air is first compressed and then cooled to liquefaction. Liquid air is then separated into its oxygen, nitrogen, and argon components using differences in boiling points. This process is very energy-intensive, with over 65% of the cost of oxygen production dependent on energy consumption. -
PSA (Pressure Swing Adsorption):
Air is selectively separated from oxygen by pressure fluctuations using an adsorbent (e.g., zeolite) PSA can yield low purity (90-95%) oxygen at relatively low energy, but if high purity oxygen is required, low temperature distillation
is recommended
. distillation method is recommended when high purity oxygen is required. - By-product utilization:
Effective utilization of nitrogen is also important because the oxygen production process also produces large amounts of nitrogen at the same time. For example, nitrogen may be utilized for cooling purposes or as a raw material in the chemical industry.
propane gas
The industrial production of propane gas (C₃H₈) is primarily accomplished by refining natural gas and refining crude oil. Propane is unique in that it is produced as a byproduct of these processes, and therefore does not require any additional special feedstock.
Below are the approximate inputs, energy consumption, and waste for producing 1 kg of propane.
Approximate input and energy for producing 1 kg of propane
Input power
| (data) item | Approximate quantity (kWh/kg C₃H₈) |
| Power (for compression and separation) | 0.2 to 0.3 kWh |
Input fuel
| type | Approximate quantity (kg) |
| Natural gas or fuel oil (for heating) | 0.1 to 0.2 kg |
Other than combustion, CO₂
| Approximate amount generated (kg CO₂/kg C₃H₈) |
| 0.01 to 0.02 kg |
input
| name | Approximate input amount (kg/kg C₃H₈) |
| Natural gas (unprocessed, main component CH₄) | 2.0 to 2.5 kg |
| Water (for cooling and separation) | 1.0 to 1.5 kg |
Solvents and other process substances
| name | Approximate amount used (kg) | Cycloparametric rate |
| Cooling water (for heat exchange) | 4-6 kg | 90-95% (circulation used) |
| Lubricants (for equipment) | 0.01 to 0.02 kg | 90-95% (circulation used) |
Waste requiring treatment
| name | Approximate amount (kg/kg C₃H₈) |
| Heavy hydrocarbon residue | 0.02 to 0.05 kg |
| Cleaning effluent (impurities contained) | 0.01 to 0.02 kg |
Process Details and Considerations
- Natural Gas Refining and Separation: During natural gas refining, propane is separated along with other components such as methane (CH₄). Propane, which is about 5% of natural gas, is extracted through a liquefaction and compression separation process. This involves compression and cooling, which consumes energy.
- Propane Production from Crude Oil Refining: During crude oil refining, propane is obtained as a byproduct along with other liquefied petroleum gas (LPG). Propane is formed during the distillation of crude oil to separate components with different molecular weights. The amount of propane produced depends on the efficiency of the refining process and the composition of the crude oil.
- Environmental and Energy Consumption Impacts: Because propane production is primarily a byproduct of other fossil fuel generation rather than a direct fuel generation, the environmental impact depends on the process of the producer. Improving the efficiency of natural gas and crude oil refining processes is critical to the sustainable production of propane.
hydrogen peroxide
Hydrogen peroxide (H₂O₂) is primarily produced using the **Anthraquinone Process**, which is suitable for mass production. Below are the approximate inputs, energy consumption, and waste for producing 1 kg of hydrogen peroxide.
Approximate input and energy for producing 1 kg of hydrogen peroxide
Input power
| (data) item | Approximate quantity (kWh/kg H₂O₂) |
| Power (for compression, mixing and separation) | 0.8 to 1.0 kWh |
Input fuel
| type | Approximate quantity (kg) |
| Natural gas or fuel oil (for heating) | 0.2 to 0.3 kg |
Other than combustion, CO₂
| Approximate amount generated (kg CO₂/kg H₂O₂) |
| 0.02 to 0.05 kg |
input
| name | Approximate amount of input (kg/kg H₂O₂) |
| Hydrogen (H₂, 99.99%) | 0.02 to 0.05 kg |
| Oxygen (O₂, 99.99%) | 0.25 to 0.3 kg |
| Anthraquinone (mediator) | 0.1-0.2 kg (reused) |
Solvents and other process substances
| name | Approximate amount used (kg) | Cycloparametric rate |
| Cooling water (for heat exchange) | 5 to 7 kg | 90-95% (circulation used) |
| Organic solvent (e.g., toluene) | 0.2 to 0.3 kg | 85-95% (circulation use) |
Waste requiring treatment
| name | Approximate quantity (kg/kg H₂O₂) |
| By-products (heavy hydrocarbons and organic waste) | 0.05 to 0.1 kg |
| Cleaning effluent (impurities contained) | 0.02 to 0.04 kg |
Process Details and Considerations
- Anthraquinone Method Overview:
In this process, anthraquinone is first reacted with hydrogen to produce anthrahydroquinone, which is then reacted with oxygen to produce hydrogen peroxide. The anthraquinone is then regenerated and the process is repeated. - Cooling and Separation Process:
The hydrogen peroxide produced is collected as an aqueous solution, cooled, and separated. Cooling water and organic solvents are used to control temperature and separate the products. - Waste Management:
Waste generated during the process (unreacted organics and heavy hydrocarbons) is often properly separated and reused, but waste disposal can be a challenge.
nitrogen (N)
Cryogenic Distillation, Pressure Swing Adsorption (PSA), and **Membrane Separation** are three common methods used to produce nitrogen (N₂) in industry. PSA, and **Membrane Separation**. Below is a rough estimate of the inputs, energy consumption, and waste to produce 1 kg of nitrogen.
Approximate input and energy required to produce 1 kg of nitrogen
Input power
| (data) item | Approximate quantity (kWh/kg N₂) |
| Power (for compression and separation) | 0.5 to 1.2 kWh |
Input fuel
| type | Approximate quantity (kg) |
| Natural gas or fuel oil (for heating) | 0.05 to 0.1 kg |
Other than combustion, CO₂
| Approximate amount generated (kg CO₂/kg N₂) |
| 0.01 to 0.02 kg |
input
| name | Approximate amount of input (kg/kg N₂) |
| Air (raw material) | 4.5 to 5.5 kg |
| Water (for cooling) | 0.8 to 1.2 kg |
Solvents and other process substances
| name | Approximate amount used (kg) | Cycloparametric rate |
| Cooling water (for heat exchange) | 5 to 7 kg | 90-95% (circulation used) |
| Lubricating oil (for equipment) | 0.01 to 0.02 kg | 90-95% (circulation used) |
Waste requiring treatment
| name | Approximate amount (kg/kg N₂) |
| Oxygen and trace impurity residue | 0.02 to 0.05 kg |
Process Details and Considerations
- Cryogenic Distillation:
A method of liquefying compressed air by cooling it and using its different boiling points (oxygen: -183°C, nitrogen: -196°C) to separate nitrogen and oxygen. It is suitable for large-scale high-purity nitrogen production and is commonly used in industrial applications. Due to its high energy consumption, it is
usually used for mass production - Pressure Swing Adsorption (PSA) method:
This method selectively adsorbs and separates oxygen by varying pressure using an adsorbent such as zeolite. This process consumes relatively little energy and is
suitable for producing nitrogen of up to 95% purity. - Membrane Separation:
This method uses a semipermeable membrane to separate nitrogen and oxygen in the air. This method is often used for small-scale production and applications that require portability, and although it is low cost, it is
not suitable for high-purity nitrogen production.
lubricating oil
The general method used to produce lubricating oil involves refining crude oil and blending the base oil. First, high-viscosity heavy oil is refined from crude oil, which is then used to produce lubricant base stock. Then, additives are added to the base oil to obtain the final product. The process includes several stages: distillation, refining, dewaxing (dewaxing), and blending of additives.
Below are the approximate inputs, energy consumption, and waste for producing 1 kg of lubricating oil.
Approximate input and energy required to produce 1 kg of lubricating oil
Input power
| (data) item | Approximate quantity (kWh/kg lubricating oil) |
| Power (for compression, distillation and separation) | 0.5 to 0.7 kWh |
Input fuel
| type | Approximate quantity (kg) |
| Natural gas or fuel oil (for heating) | 0.15 to 0.25 kg |
Other than combustion, CO₂
| Approximate amount generated (kg CO₂/kg lubricating oil) |
| 0.03 to 0.05 kg |
input
| name | Approximate input amount (kg/kg lubricating oil) |
| Crude oil (heavy fractional distillate oil) | 1.5 to 2.0 kg |
| Additives (e.g., antioxidants, viscosity improvers) | 0.02 to 0.05 kg |
| Solvent (e.g., furfural, acetone) | 0.1 to 0.2 kg |
Solvents and other process substances
| name | Approximate amount used (kg) | Cycloparametric rate |
| Cooling water (for heat exchange) | 4-6 kg | 85-95% (circulation use) |
| Lubricants (for equipment) | 0.01 to 0.02 kg | 90-95% (circulation used) |
Waste requiring treatment
| name | Approximate quantity (kg/kg lubricant) |
| Unreacted heavy residue | 0.05 to 0.1 kg |
| Waste solvent and impurity sludge | 0.02 to 0.04 kg |
Process Details
- Distillation and Refining:
Heavy oils are extracted from crude oil and distilled to produce base stocks for various lubricants. Heavy oils are typically vacuum distilled to obtain fractions with different molecular weights and boiling point ranges. Subsequent processes such as dewaxing and hydrogenation refinement are used to remove impurities and improve product quality. - Solvent Use and Regeneration:
Organic solvents such as furfural and acetone are used in solvent refining and dewaxing processes. These solvents are typically recovered and reused in the manufacturing process. - Additive blending:
Base oils are blended with appropriate additives (e.g., antioxidants, viscosity improvers, etc.) to produce a purpose-built end product. Additive ratios vary depending on the application and adjust the performance of various products such as engine oil, gear oil, and industrial lubricants.
Polystyrene Ion Exchange Resins
The approximate inputs, energy consumption, and waste for the production of 1 kg of polystyrene-based Ion Exchange Resin (Polystyrene-based Ion Exchange Resin) are as follows. The process mainly involves using polystyrene as a base material and introducing functional groups (e.g., sulfonic acid and amino groups) to give it an ion exchange function.
Approximate input and energy for producing 1 kg of polystyrene-based ion exchange resin
Input power
| (data) item | Approximate quantity (kWh/kg resin) |
| Power (for mixing, reaction and separation) | 1.2 to 1.5 kWh |
Input fuel
| type | Approximate quantity (kg) |
| Natural gas or fuel oil (for heating) | 0.1 to 0.2 kg |
Other than combustion, CO₂
| Approximate amount generated (kg CO₂/kg resin) |
| 0.02 to 0.04 kg |
input
| name | Approximate input amount (kg/kg resin) |
| Styrene monomer (Styrene) | 0.5 to 0.6 kg |
| Divinylbenzene (DVB, cross-linking agent) | 0.1 to 0.2 kg |
| Solvent (e.g., toluene) | 0.15 to 0.25 kg |
| Sulfuric acid (H₂SO₄, for introduction of functional groups) | 0.05 to 0.1 kg |
| Water (for cooling and cleaning) | 1.5 to 2.0 kg |
Solvents and other process substances
| name | Approximate amount used (kg) | Cycloparametric rate |
| Cooling water (for heat exchange) | 4-6 kg | 85-90% (circulation used) |
| Organic solvent (e.g., toluene, xylene) | 0.1 to 0.2 kg | 70-85% (circulation used) |
Waste requiring treatment
| name | Approximate quantity (kg/kg resin) |
| Unreacted monomers and organic sludge | 0.02 to 0.05 kg |
| Acidic liquid waste (byproduct of functional group introduction) | 0.02 to 0.04 kg |
Manufacturing Process Details and Considerations
- Polystyrene Synthesis and Crosslinking:
Polystyrene monomer and divinylbenzene are used to polymerize polystyrene. Divinylbenzene is used as a cross-linking agent to improve the stability and mechanical properties of the resin. Organic solvents are used in the polymerization reaction, and the solvents are recovered and reused after the reaction. - Introduction of functional groups:
Sulfonic acid and amino groups are introduced to the surface of polymerized polystyrene using acids and bases to provide an ion exchange function. This process uses chemicals such as sulfuric acid and hydrochloric acid and generates waste acid. - Separation and Purification:
After functional group introduction, the resin undergoes a cooling and washing process to remove impurities. This process uses large volumes of water, which is often treated as process wastewater. Cooling water and washing solvents are typically circulated.
Polystyrene Ion Exchange Resins
The functional groups used to produce 1 kg of Polystyrene-based Ion Exchange Resin are obtained by introducing specific chemical groups to give it ion exchange properties. Below is a rough estimate of inputs, energy consumption, and waste including these functional groups and the process used to produce them.
Approximate input and energy for producing 1 kg of polystyrene-based ion exchange resin
Input power
| (data) item | Approximate quantity (kWh/kg resin) |
| Power (for mixing, reaction and separation) | 1.2 to 1.5 kWh |
Input fuel
| type | Approximate quantity (kg) |
| Natural gas or fuel oil (for heating) | 0.1 to 0.2 kg |
Other than combustion, CO₂
| Approximate amount generated (kg CO₂/kg resin) |
| 0.02 to 0.04 kg |
input
| name | Approximate input amount (kg/kg resin) |
| Styrene monomer (Styrene) | 0.5 to 0.6 kg |
| Divinylbenzene (DVB, cross-linking agent) | 0.1 to 0.2 kg |
| Methylene chloride (solvent for introducing functional groups) | 0.1 to 0.15 kg |
| Chloromethyl group (functional group introduction) | 0.05 to 0.1 kg |
| Sulfonic acid group (for cation exchange) | 0.02 to 0.05 kg |
| Ammonium group (for anion exchange) | 0.02 to 0.04 kg |
| Water (for cooling and cleaning) | 1.5 to 2.0 kg |
Solvents and other process substances
| name | Approximate amount used (kg) | Cycloparametric rate |
| Cooling water (for heat exchange) | 4-6 kg | 85-90% (circulation used) |
| Organic solvent (e.g., toluene, chloromethyl) | 0.1 to 0.2 kg | 70-85% (circulation used) |
Waste requiring treatment
| name | Approximate quantity (kg/kg resin) |
| Unreacted monomers and organic sludge | 0.02 to 0.05 kg |
| Acidic liquid waste (byproduct of functional group introduction) | 0.02 to 0.04 kg |
Introduction of functional groups in the manufacturing process
- Introduction of sulfonic
acid groups (for cation exchange resins)
Sulfonation reactions are performed on styrene-divinylbenzene (DVB) copolymers to introduce sulfonic acid groups (-SO₃H). This yields a strongly acidic cation exchange resin that can be used under a wide range of pH conditions. - Introduction of chloromethyl and ammonium groups (for anion exchange resins)
After adding chloromethyl groups to the styrene-DVB copolymer, strongly or weakly basic ammonium groups (-NR₄⁺) are introduced by
amination. This makes it suitable for use as an anion exchange resin, for pH adjustment and removal of organic acids.
These values are a guide based on a typical manufacturing process. Since they may vary depending on the manufacturing conditions and the type of chemicals used, adjustments must be made for each condition when designing detailed processes.
flake load
The amount of stripped soil in iron ore mining varies depending on the mining method and the nature of the deposit, but is generally several to several dozen times the amount of iron ore. The amount of soil stripped is generally larger than the amount of ore because surface soil and rock must be scraped away in order to extract the iron ore.
The amount of soil stripped in gold ore mining depends on the nature of the deposit and the mining method, but is generally several to several dozen times the amount of ore. In order to extract ore containing gold, it is necessary to remove surrounding rocks and soil. Therefore, the amount of soil stripped is generally larger than the amount of ore.
Below is a list of ores used for mining the major metals and elements and the approximate grades they contain. However, since grades vary by ore deposit and mining area, they should be taken as general guidelines.
- Iron: Mined from hematite (Fe2O3) and magnetite (Fe3O4), with grades generally above 50%.
- Copper: mined from brass ores (CuFeS2) and copper sulfide ores (Cu2S) with grades generally ranging from 1-5%, but sometimes higher.
- Aluminum: Mined from bauxite (Al2O3-nH2O), grades are typically 30-60%.
- Lead: mined from lead ore (PbS) and lead-zinc sulfide ore ((Pb, Zn)S), with grades generally ranging from 2-10%.
- Zinc: Mined from zinc sulfide ore (ZnS) and rhyozinc ore (ZnCO3), with grades typically ranging from 4-10%.
- Gold: mined from gold ores (gold sulfides and oxides) with grades generally around 0.5-5 g/ton, but higher grades are possible in higher grade deposits.
- Silver: Mined from silver ores (silver sulfides and oxides), grades generally range from 10-300 g/ton, although higher grades are available.
- Nickel: Mined from pentlandite ((Ni, Fe)9S8) and nickel sand ore ((Ni(Fe)2O4), with grades generally ranging from 1-3%.
The above is a general guide to ores used for mining metals and elements and the grades they contain, but may vary by mining site or region.
Below are the names of major ores and their approximate contents of boron, fluorine, sodium, magnesium, phosphorus, and sulfur.
- Boron: Borax (borate) – content typically around 10-30%.
- Fluorine: Fluorite (calcium fluoride) – content typically around 50-70%.
- Sodium: Rock salt (sodium chloride) – 100% content.
- Magnesium: LWM (magnesium carbonate) – content typically around 40-50%.
- Phosphorus: Phosphate (calcium phosphate) – content typically around 10-20%.
- Sulfur: sulfur ore (iron sulfide) – content typically around 60-70%.
Below are the main ores of K, Ca, Sc, Ti, V, Cr, Mn, Co, Ga, Ge, As, and Se and their approximate contents.
- K (potassium): potassium salt ore (potassium salts) – content typically around 20-60%.
- Ca (calcium): limestone (calcium carbonate) – content typically around 50-70%.
- Sc (scandium): scandium occurs primarily as a byproduct, and there are no examples of ores containing scandium.
- Ti (titanium): rutile (titanium dioxide) or ilmenite (iron titanium oxide) – generally 30-70% content.
- V (Vanadium): Vanadium is produced primarily as a byproduct, and there are no examples of ores containing vanadium.
- Cr (Chromium): Chromite (chromium oxide iron ore) – content generally around 40-50%.
- Mn (manganese): pyrolusite (manganese oxide ore) – content generally around 40-50%.
- Co (cobalt): electrolytic ore (arsenopyrite) – content generally around 1-5%.
- Ga (Gallium): Gallium is produced primarily as a byproduct; there are no examples of ores containing gallium.
- Ge (Germanium): Germanium is produced primarily as a byproduct; there are no examples of ores containing germanium.
- As (arsenic): arsenopyrite (arsenic copper ore) – content typically around 10-30%.
- Se (Selenium): Selenite (silver oxide ore) – generally around 1-5% content.
Below are the main ores of Rb, Sr, Y, Zr, Nb, Mo, Cd, In, Sn, Sb, Te, Cs, Ba, Hf, Ta, Re, Bi, and U and their approximate contents.
- Rb (rubidium): Rubidium occurs primarily as a byproduct and there are no examples of ores containing rubidium.
- Sr (strontium): celestite (strontium nitrate) – content typically around 60-70%.
- Y (yttrium): monazite (yttrium carbonate) – typically 30-50% content.
- Zr (zirconium): zircon (silicate mineral) – content typically around 50-60%.
- Nb (niobium): columbite (niobium oxide ore) – content typically around 40-60%.
- Mo (molybdenum): molybdenum ore (molybdenum sulfide) – generally around 0.1-1% content.
- Cd (cadmium): sphalerite (cadmium sulfide) – content typically around 50-70%.
- In (indium): sphalerite (cadmium sulfide) – Indium is produced as a byproduct of cadmium.
- Sn (tin): cassiterite (tin oxide ore) – content typically around 10-50%.
- Sb (antimony): stibnite (antimony sulfide) – content typically around 60-70%.
- Te (tellurium): calaverite (lead oxide ore) – typically 1-5%.
- Cs (cesium): Cesium is produced primarily as a byproduct; there are no examples of ores containing cesium.
- Ba (barium): barium ore (barium sulfate) – content generally around 60-70%.
- Hf (hafnium): zircon (a silicate mineral) – hafnium is a byproduct of zircon.
- Ta (tantalum): Tantalum ore (tantalum oxide ore) – content generally around 20-40%.
- Re (rhenium): molybdenum ore (molybdenum sulfide) – rhenium is a byproduct of molybdenum.
- Bi (bismuth): bismuth ore (bismuth sulfide) – content typically around 50-60%.
- U (uranium): uranium ore (uranium oxide ore) – generally contains about 0.1-1%.
Below is a rough estimate of the amount of soil excavated per kilogram for each metal. Note that these are general figures and may vary depending on the specific deposit and mining method.
- K (Potassium): To obtain 1 kg of potassium from about 0.5 – 2 kg of ore, 1 – 4 kg of soil must be excavated.
- Ca (calcium): To obtain 1 kg of calcium from about 1 – 2 kg of limestone, 1 – 2 kg of soil must be excavated.
- Sc (Scandium): There are no specific soil drilling quantities for scandium, as it is primarily a by-product.
- Ti (Titanium): To obtain 1 kg of titanium from about 1 – 3 kg of ore, 1 – 3 kg of soil must be excavated.
- V (Vanadium): Vanadium is produced primarily as a by-product and there are no specific soil drilling quantities.
- Cr (Chromium): To obtain 1 kg of chromium from approximately 2 – 5 kg of ore, 2 – 5 kg of soil must be excavated.
- Mn (manganese): To obtain 1 kg of manganese from about 1 – 3 kg of ore, 1 – 3 kg of soil must be excavated.
- Co (cobalt): To obtain 1 kg of cobalt from about 10 – 50 kg of ore, 10 – 50 kg of soil must be excavated.
- Ga (Gallium): Gallium is produced primarily as a by-product, so there is no specific soil drilling volume.
- Ge (Germanium): There are no specific soil drilling quantities for germanium, as it is produced primarily as a by-product.
- As (arsenic): To obtain 1 kg of arsenic from about 1 – 3 kg of ore, 1 – 3 kg of soil must be excavated.
- Se (Selenium): To obtain 1 kg of selenium from about 10 – 50 kg of ore, 10 – 50 kg of soil must be excavated.
These figures are only a guide and may vary depending on the actual mining location and method.
Below are general guidelines for the ratio of the amount of ore drilled to the amount of ore in the mine from which each element is obtained, as well as the grade of the ore. Note that these are general figures and may vary depending on the specific ore deposit and mining method.
- Fluorine: drilling volume > ore volume, ore grade typically around 1-5%.
- Sodium: drilling volume > ore volume, ore grade typically around 1-3%.
- Magnesium: drilling volume > ore volume, ore grade typically around 2-5%.
- Phosphorus: drilling volume > ore volume, ore grade typically around 10-20%.
- Sulfur: drilling volume > ore volume, ore grade typically around 5-20%.
- K (potassium): drilling volume > ore volume, ore grade typically around 10-30%.
- Ca (calcium): drilling volume > ore volume, ore grade typically around 40-60%.
- Ti (titanium): drilling volume > ore volume, ore grade typically around 1-10%.
- Cr (chromium): drilling volume > ore volume, ore grade typically around 20-40%.
- Mn (manganese): drilling volume > ore volume, ore grade typically around 10-30%.
- Co (cobalt): drilling volume > ore volume, ore grade typically around 0.1-0.5%.
- As (arsenic): drilling volume > ore volume, ore grades typically around 1-5%.
- Se (selenium): drilling volume > ore volume, ore grade typically around 0.1-1%.
- Sr (strontium): drilling volume > ore volume, ore grade typically around 60-70%.
- Y (yttrium): drilling volume > ore volume, ore grade typically 30-50%.
- Zr (zirconium): drilling volume > ore volume, ore grade typically around 50-60%.
- Nb (niobium): drilling volume > ore volume, ore grade typically around 40-60%.
- Mo (molybdenum): drilling volume > ore volume, ore grade typically around 0.1-1%.
- Cd (cadmium): drilling volume > ore volume, ore grade typically around 50-70%.
- Sn (tin): drilling volume > ore volume, ore grade typically around 10-50%.
- Sb (antimony): drilling volume > ore volume, ore grade typically around 60-70%.
- Te (tellurium): drilling volume > ore volume, ore grade typically around 1-5%.
- Ba (barium): drilling volume > ore volume, ore grade typically around 60-70%.
- Ta (tantalum): drilling volume > ore volume, ore grade typically around 20-40%.
- Bi (bismuth): drilling volume > ore volume, ore grade typically around 50-60%.
- U (uranium): drilled > ore volume, ore grade typically around 0.1-1%.
- Fluorine: drilling volume > ore volume, ore grade typically 1-5%; to obtain 1 kg of fluorine, dozens of kg of ore must be drilled.
- Sodium: drilling volume > ore volume; ore grade is typically around 1-3%; to obtain 1 kg of sodium, dozens of kg of ore must be drilled.
- Magnesium: drilling volume > ore volume, ore grade typically around 2-5%; to obtain 1 kg of magnesium, dozens of kg of ore must be drilled.
- Phosphorus: drilling volume > ore volume, ore grade typically around 10-20%; to obtain 1 kg of phosphorus, dozens of kg of ore must be drilled.
- Sulfur: drilling volume > ore volume, ore grade typically around 5-20%; to obtain 1 kg of sulfur, dozens of kg of ore must be drilled.
- K (potassium): drilling volume > ore volume, ore grade typically around 10-30%; to obtain 1 kg of potassium, dozens of kg of ore must be drilled.
- Ca (calcium): drilling volume > ore volume; ore grade is typically 40-60%; several tens of kilograms of ore must be drilled to obtain 1 kg of calcium.
- Ti (titanium): drilling volume > ore volume; ore grade is typically 1-10%; several tens of kilograms of ore must be drilled to obtain 1 kg of titanium.
- Cr (chromium): drilling volume > ore volume; ore grade is typically around 20-40%; several tens of kilograms of ore must be drilled to obtain 1 kg of chromium.
- Mn (manganese): drilling volume > ore volume; ore grade is typically around 10-30%; to obtain 1 kg of manganese, tens of kilograms of ore must be drilled.
- Co (cobalt): drilling volume > ore volume, ore grade generally around 0.1-0.5%; several hundred kg of ore must be drilled to obtain 1 kg of cobalt.
- As (arsenic): drilling volume > ore volume; ore grade is typically 1-5%; several tens of kilograms of ore must be drilled to obtain 1 kg of arsenic.
- Se (selenium): drilling volume > ore volume; ore grade is typically around 0.1-1%; several hundred kilograms of ore must be drilled to obtain 1 kg of selenium.
- Sr (strontium): drilling volume > ore volume; ore grade is typically 60-70%; several kilograms of ore must be drilled to obtain 1 kg of strontium.
- Y (yttrium): drilling volume > ore volume; ore grade is typically 30-50%; several kilograms of ore must be drilled to obtain 1 kg of yttrium.
- Zr (zirconium): drilling volume > ore volume; ore grade is typically 50-60%; several kilograms of ore must be drilled to obtain 1 kg of zirconium.
- Nb (niobium): drilling volume > ore volume; ore grade is typically 40-60%; several kilograms of ore must be drilled to obtain 1 kg of niobium.
- Mo (molybdenum): drilling volume > ore volume; ore grade is typically around 0.1-1%; several hundred kilograms of ore must be drilled to obtain 1 kg of molybdenum.
- Cd (cadmium): drilling volume > ore volume; ore grade is typically 50-70%; several kilograms of ore must be drilled to obtain 1 kg of cadmium.
- Sn (tin): drilling volume > ore volume; ore grade is typically 10-50%; several kilograms of ore must be drilled to obtain 1 kg of tin.
- Sb (antimony): drilling volume > ore volume; ore grade is typically around 60-70%; several kilograms of ore must be drilled to obtain 1 kg of antimony.
- Te (tellurium): drilling volume > ore volume; ore grade is typically 1-5%; to obtain 1 kg of tellurium, dozens of kg of ore must be drilled.
- Ba (barium): drilling volume > ore volume; ore grade is typically around 60-70%; several kilograms of ore must be drilled to obtain 1 kg of barium.
- Ta (tantalum): drilling volume > ore volume; ore grade is typically around 20-40%; several tens of kilograms of ore must be drilled to obtain 1 kg of tantalum.
- Bi (bismuth): drilling volume > ore volume; ore grade is typically 50-60%; several kilograms of ore must be drilled to obtain 1 kg of bismuth.
- U (uranium): drilling volume > ore volume, ore grade is typically around 0.1-1%; to obtain 1 kg of uranium, several hundred kg of ore must be drilled.
The amount of concrete and rebar input and energy used to dig mining tunnels in pitmining (open pit mining) in mines depends on the type of mineral and deposit, geological conditions, and mining method. As a general guideline, 0.1 to 0.3 cubic meters of concrete and 10 to 30 kilograms of rebar are considered per ton of mineral to be mined. As for the amount of energy, the energy required for concrete production and tunnel excavation must be taken into account, but a typical range is from a few hundred kilowatt hours to several megawatt hours.
The amount of concrete and rebar and energy used to dig tunnels for mining in mountain underground mining depends on the type of mineral to be mined, geological conditions, and mining method. As a general guideline, 0.3 to 1.0 cubic meters of concrete and 30 to 100 kilograms of rebar are considered per ton of mined mineral. As for the amount of energy, the energy required for concrete production and tunnel excavation must be taken into account, but a typical range is from a few hundred kilowatt hours to a few megawatt hours.
bonding agent
Although different processes are used to manufacture bonding agents depending on their chemical composition and application, the following is an example of an industrially common manufacturing process and a guide to its inputs and energy.
Inputs and energy guidelines for bonding agent production process
- energy input
- Power input: 1.5 to 2.5 kWh/kg (reactor, agitator, temperature control)
- Input Fuel: Fuel
- Type: Natural Gas
- Quantity: 0.3-0.5 kg/kg (for drying and heating processes)
- CO₂ emissions other than combustion
- CO₂ emissions: 0.2-0.5 kg/kg (solvent volatilization, byproducts from chemical reactions)
- raw material input
- Name | Approximate amount of dosage
- Polyol | 0.4-0.6 kg
- Isocyanate | 0.3-0.5 kg
- Additives (hardener, stabilizer) | 0.05-0.1 kg
- process solvents and auxiliaries
- Name | Usage | Circulation Rate
- Toluene | 0.2~0.4 kg | 80~90
- Acetone | 0.1-0.2 kg | 85-95
- amount and type of waste disposed
- Effluent | 0.3-0.5 kg/kg (solvent washing and reaction effluent)
- Solid waste | 0.1-0.2 kg/kg (filter cake, catalyst residue)
- changes in natural resources (e.g., water, soil)
- Water consumption: 2-5 kg/kg (cooling and cleaning water)
- Soil and ore: No impact (typically not used in the manufacturing phase)
Manufacturing Process Points
- Temperature control: Chemical reactions must be controlled and require constant heating and cooling.
- Volatile Organic Compounds (VOCs): Solvent recovery systems are often used to control emissions.
- Waste Disposal: Waste water is usually attempted to be reused by neutralization or distillation.
nitric acid
The **ammonia oxidation process (Ostwald process)** is commonly used for the industrial production of nitric acid (HNO₃). The following are approximate values for the energy and resource input for this process.
Inputs and approximate energy for 1 kg nitric acid production
- energy input
- Power input: 0.4-0.6 kWh/kg (used for compression and reactor control)
- Input Fuel: Fuel
- Type: Natural Gas
- Quantity: 0.05 to 0.1 kg/kg (for process heating)
- CO₂ emissions other than combustion
- CO₂ emissions: 0.02-0.05 kg/kg (CO₂ generated incidentally in the process)
- raw material input
- Name | Approximate amount of dosage
- Ammonia (NH₃) | 0.23-0.25 kg
- Air (oxygen source) | 1.2 to 1.5 kg (for oxidation process)
- Water | 0.5 to 1 kg (for generating nitric acid solution)
- process solvents and auxiliaries
- Name | Usage | Circulation Rate
- Cooling water | 3-5 kg | 90-95% (for heat exchange)
- Catalyst (platinum-based catalyst) | 0.01-0.02 kg | Almost 100% reusable
- amount and type of waste disposed
- Waste gas | 0.1-0.2 kg/kg (unreacted gas and byproducts)
- Some nitrogen oxides (NOx) are recycled in the recovery facility.
- Solid waste | None (very little in normal production)
- changes in natural resources (e.g., water, soil)
- Water consumption: 3-6 kg/kg (process cooling and produced water)
- Soil and ore effects: None (nitric acid production processes usually do not affect them)
Process Overview
- Ammonia oxidation: Ammonia reacts with air to produce nitric oxide (NO).
- Oxidation and absorption: The NO produced is further oxidized and the nitrogen oxide is absorbed by water to obtain a nitric acid solution.
- Cooling and Concentration: The nitric acid solution is cooled and concentrated if necessary.
Points to keep in mind when manufacturing
- Control of NOx gas emissions is important, and recovery is performed by exhaust gas treatment equipment.
- The heat exchange system recovers energy and reduces fuel use.
- The platinum catalyst is carefully controlled to prevent catalyst degradation.
Although this process is highly efficient, managing the NOx and CO₂ emissions is an environmental challenge.
flotation agent
Flotation agent (flotation reagent) 1 kg Inputs and energy requirements for production
Flotation agents are widely used in the ore flotation process, especially** xanthates (Xanthates)** are common reagents. This reagent is used to recover sulfide minerals, of which sodium xanthate (Sodium Xanthate) is a typical industrial example.
- energy input
- Power input: 0.5-0.8 kWh/kg
(used for reaction process and drying process) - Input Fuel: Fuel
- Type: Natural Gas
- Quantity: 0.1 to 0.2 kg/kg
(for drying and heating processes)
- CO₂ emissions other than combustion
- CO₂ emissions: 0.03-0.06 kg/kg
(CO₂ generated as a side effect from chemical reactions)
- raw material input
- Name | Approximate amount of dosage
- Ethanol (C₂H₅OH) | 0.2-0.4 kg
- Carbon disulfide (CS₂) | 0.5-0.6 kg
- Caustic soda (NaOH) | 0.1 to 0.15 kg
- Water | 0.5 to 0.7 kg
- process solvents and auxiliaries
- Name | Usage | Circulation Rate
- Cooling water | 3-5 kg | 90-95% (cooling process)
- Acetone | 0.05-0.1 kg | 80-90% (cleaning process)
- amount and type of waste disposed
- Effluent | 0.3-0.4 kg/kg
(solvent residue and washing water) - Organic waste | 0.05-0.1 kg/kg (unreacted materials and byproducts)
- changes in natural resources (e.g., water, soil)
- Water consumption: 3-6 kg/kg
(for process cooling and cleaning) - Soil and ore effects: None (not used in normal production)
Manufacturing Process Overview
- Reaction: Ethanol reacts with carbon disulfide to produce xanthogenate.
- Neutralization: Neutralize the reaction product with caustic soda to obtain sodium xanthogenate.
- Drying: The resulting solution is concentrated and dried to produce a flotation agent in powder form.
Points to keep in mind when manufacturing
- Control of CS₂: Carbon disulfide is toxic and requires strict handling.
- VOC emission control: Since volatile solvents are used, it is recommended that a recovery system be installed.
- Wastewater treatment: Generated wastewater must be properly treated to meet environmental standards.
The process has relatively low energy consumption, but the use of CS₂ is an environmental and safety challenge. Emission control of volatile compounds is a key aspect of the production process.
propionaldehyde
Propionaldehyde (CH₃CH₂CHO) is produced primarily by the oxidation of propylene. The oxidation process uses a catalyst and oxygen to selectively oxidize propylene to form aldehydes.
- energy input
- Power input: 0.7 to 1.0 kWh/kg
(temperature control of compression and reaction processes) - Input Fuel: Fuel
- Type: Natural Gas
- Volume: 0.1 to 0.15 kg/kg
(for heating and distillation processes)
- CO₂ emissions other than combustion
- CO₂ emissions: 0.02-0.05 kg/kg
(CO₂ as byproduct of oxidation reaction)
- raw material input
- Name | Approximate amount of dosage
- Propylene (C₃H₆) | 0.75-0.85 kg
- Oxygen (O₂) | 0.25 to 0.3 kg
- Catalyst (molybdenum oxide catalyst) | 0.01 to 0.02 kg
- process solvents and auxiliaries
- Name | Usage | Circulation Rate
- Cooling water | 4-6 kg | 90-95% (for reaction cooling)
- Solvent for distillation (e.g., acetone) | 0.1-0.2 kg | 85-90% (purification process)
- amount and type of waste disposed
- Effluent | 0.2-0.3 kg/kg (residual liquid from washing and unreacted materials)
- Waste gas | 0.05-0.1 kg/kg (unreacted propylene and byproducts)
- changes in natural resources (e.g., water, soil)
- Water consumption: 5-8 kg/kg (cooling and cleaning processes)
- Soil and ore impact: None (not used directly in the manufacturing process)
Manufacturing Process Overview
- Propylene oxidation: Propylene and oxygen are oxidized using a molybdenum oxide catalyst to form propionaldehyde.
- Distillation and purification: The product is distilled to obtain highly pure propionaldehyde.
- Waste treatment: Recovery and treatment of by-products and unreacted materials.
Points to keep in mind when manufacturing
- Reaction conditions: Control of temperature and pressure is important to achieve selective oxidation.
- VOC management: A system is needed to collect unreacted and waste products of the reacted gases.
- Catalyst regeneration: Molybdenum-based catalysts can be regenerated and are expected to last for a long time.
In the production of propionaldehyde, energy efficiency and catalyst management are key to reducing production costs. Since distillation is required in the purification process, process optimization to reduce energy consumption is important.
Methacrylic acid
Methacrylic acid (MAA) is industrially used in the acetone cyanohydrin process and in the oxidation of isobutylene. Here, a guide is given based on the oxidation process of isobutylene.
- energy input
- Power input: 0.8-1.2 kWh/kg
(used for agitation, reaction temperature control, and compression processes) - Input Fuel: Fuel
- Type: Natural Gas
- Volume: 0.15-0.25 kg/kg
(used in distillation and drying processes)
- CO₂ emissions other than combustion
- CO₂ emissions: 0.02-0.06 kg/kg
(CO₂ generated by side reactions)
- raw material input
- Name | Approximate amount of dosage
- Isobutylene (C₄H₈) | 0.6-0.7 kg
- Oxygen (O₂) | 0.3 to 0.5 kg
- Water | 0.4 to 0.6 kg
- Catalyst (molybdenum-vanadium catalyst) | 0.01 to 0.02 kg
- process solvents and auxiliaries
- Name | Usage | Circulation Rate
- Cooling water | 5 to 7 kg | 90 to 95
- Acetone | 0.05-0.1 kg | 85-95% (used for cleaning)
- amount and type of waste disposed
- Effluent | 0.3-0.5 kg/kg
(unreacted material, washing effluent) - Waste gas | 0.05-0.1 kg/kg (unreacted gas, byproducts)
- changes in natural resources (e.g., water, soil)
- Water consumption: 4-8 kg/kg
(used in cooling and reaction processes) - Soil and ore impact: None (not used directly in the manufacturing process)
Manufacturing Process Overview
- Oxidation reaction: Isobutylene is oxidized with oxygen over a molybdenum-vanadium catalyst to produce methacrylic acid.
- Distillation and Purification: The product is distilled to increase purity.
- Waste Disposal: Unreacted gas and liquid wastes are properly collected and disposed of.
Points to keep in mind when manufacturing
- Temperature control of oxidation reactions: Control of temperature and reaction time is important to prevent over-oxidation.
- Catalyst reuse: Catalysts are renewable and long-term use reduces costs.
- Waste Disposal: Liquid and gaseous waste is collected for reuse or neutralization.
The process is relatively energy efficient, but requires management of waste gases and byproducts. Regeneration of the catalyst and reuse of the cooling water reduces costs and environmental impact.
Sodium sulfate (Na₂SO₄)
Sodium sulfate is produced by the Mannheim process (reaction of sodium chloride with sulfuric acid) or by recovery as a byproduct. Here is a rough estimate of the input and energy for production by the typical Mannheim process.
- energy input
- Power input: 0.4-0.6 kWh/kg
(used for reactor, agitator, and conveying equipment) - Input Fuel: Fuel
- Type: Natural Gas
- Quantity: 0.1 to 0.15 kg/kg
(for heating processes in reactors)
- CO₂ emissions other than combustion
- CO₂ emissions: 0.02-0.04 kg/kg
(from chemical reactions and byproducts)
- raw material input
- Name | Approximate amount of dosage
- Sodium chloride (NaCl) | 0.8 to 1.0 kg
- Sulfuric acid (H₂SO₄) | 0.6-0.8 kg
- process solvents and auxiliaries
- Name | Usage | Circulation Rate
- Cooling water | 4-6 kg | 90-95
- Cleaning water | 0.2~0.5 kg | 85~90
- amount and type of waste disposed
- Waste gas | 0.1-0.2 kg/kg (by-product HCl gas)
- Can be recovered and reused as hydrochloric acid (HCl).
- Effluent | 0.05-0.1 kg/kg (cleaning effluent)
- changes in natural resources (e.g., water, soil)
- Water consumption: 5-8 kg/kg
(used for cooling and cleaning processes) - Soil and ore impact: None (not used directly in the manufacturing process)
Manufacturing Process Overview
- Mannheim reaction: Sodium chloride and sulfuric acid react at high temperatures (600-700°C) to produce sodium sulfate and hydrogen chloride gas.
- Cooling and Purification: The sodium sulfate produced is cooled and recovered as a solid.
- Waste gas treatment: By-product HCl gas is recovered and reused as hydrochloric acid.
Points to keep in mind when manufacturing
- Control of high temperature reactions: Control of reaction temperatures is important and overheating must be avoided.
- Recovery of HCl gas: Reduces environmental impact by properly recovering and reusing waste gas.
- Cooling water reuse: Cost savings can be realized by increasing the cooling water circulation rate.
The process is relatively simple, with the key points being the maintenance of reaction temperature and the treatment of the byproduct, hydrochloric acid. Environmental impact can be reduced by reusing cooling water and effectively operating a waste gas recovery system.
benzene-sulfonic acid
Benzene sulfonic acid is produced by the sulfonation reaction of benzene and concentrated sulfuric acid. In this process, concentrated sulfuric acid and fuming sulfuric acid are used as sulfonating agents, and the reaction proceeds in a high-temperature environment.
- energy input
- Power input: 0.5-0.8 kWh/kg
(used for agitation, temperature control, and pumping equipment) - Input Fuel: Fuel
- Type: Natural Gas
- Volume: 0.1-0.2 kg/kg
(for maintaining reaction temperature and drying process)
- CO₂ emissions other than combustion
- CO₂ emissions: 0.02-0.05 kg/kg
(CO₂ as a byproduct of chemical reactions)
- raw material input
- Name | Approximate amount of dosage
- Benzene (C₆H₆) | 0.7-0.8 kg
- Fuming sulfuric acid (Oleum) | 1.0-1.2 kg
- process solvents and auxiliaries
- Name | Usage | Circulation Rate
- Cooling water | 5-7 kg | 90-95% (for reaction cooling process)
- Cleaning water | 0.3-0.5 kg | 85-90% (used for equipment cleaning)
- amount and type of waste disposed
- Effluent | 0.4-0.6 kg/kg
(residual liquid after reaction or washing water) - Gas waste | 0.1-0.2 kg/kg (unreacted gas and sulfuric acid fog)
- changes in natural resources (e.g., water, soil)
- Water consumption: 6-10 kg/kg
(used for cooling and cleaning processes) - Soil and ore impact: None (not used directly in the manufacturing process)
Manufacturing Process Overview
- Sulfonation reaction: Benzene reacts with fuming sulfuric acid to produce benzene sulfonic acid.
- Post-reaction neutralization: If necessary, neutralize residual sulfuric acid with alkali.
- Washing and drying: The product is washed to remove impurities and then dried.
Points to keep in mind when manufacturing
- Temperature control: Reactions proceed at appropriate temperatures to reduce side reactions.
- Sulfuric Acid Effluent Treatment: Effluent treatment is critical, and the use of a recovery system reduces costs and environmental impact.
- Cooling water reuse: Increases energy efficiency by increasing the rate of cooling water circulation.
Since this process uses highly concentrated sulfuric acid, proper treatment of the effluent and prevention of equipment corrosion are important. Reuse of cooling water and recovery of byproducts can reduce costs and environmental impact.
soya bean (soybean)
Soybean production is dependent on agricultural activities, which primarily include field plowing, fertilization, irrigation, harvesting, and other processes. Below is a rough estimate of inputs and energy for a typical commercial-scale soybean operation.
- energy input
- Power input: 0.05-0.2 kWh/kg
(irrigation systems, use of farm machinery) - Input Fuel: Fuel
- Type: Diesel oil or biofuel
- Quantity: 0.1 to 0.15 kg/kg
(for tractor and harvester operations)
- CO₂ emissions other than combustion
- CO₂ emissions: 0.01-0.03 kg/kg
(greenhouse gas emissions from fertilizer use and microbial activity)
- raw material input
- Name | Approximate amount of dosage
- Seeds | 0.01 to 0.02 kg
- Nitrogen fertilizer (N) | 0.05-0.1 kg
- Phosphate fertilizer (P₂O₅) | 0.03-0.05 kg
- Potash fertilizer (K₂O) | 0.02-0.04 kg
- process solvents and auxiliaries
- Name | Usage | Circulation Rate
- Irrigation water | 2-5 kg | mostly consumed (no reuse)
- Pesticides (herbicides and insecticides) | 0.005-0.01 kg | Decompose or discharge after use
- amount and type of waste disposed
- Waste | Approximate Amount
- By-products (stems, leaves, etc.) | 0.2-0.4 kg/kg (reused as fertilizer or feed)
- Waste pesticide containers | 0.001~0.002 kg/kg (properly collected and disposed)
- changes in natural resources (e.g., water, soil)
- Water consumption: 3,000-5,000 L/kg (irrigation water)
- Soil effects: Nitrogen is depleted from the soil, requiring crop rotation and fertilizer application in some cases
Manufacturing Process Overview
- Cultivating and seeding: Tractors are used to plow and seed the fields.
- Fertilization and irrigation: Apply nitrogen and phosphate fertilizers and water as needed.
- Management and weeding: Use herbicides and insecticides to reduce the impact of pests and weeds.
- Harvesting: Soybeans are harvested using combine harvesters and other machinery.
Points to keep in mind when manufacturing
- Optimize fertilizer use: Over-fertilization increases environmental impact, so optimization is important.
- Management of water resources: Irrigation should increase the efficiency of water resource use.
- Residue utilization: Stems and leaves can be reused as compost or fodder after harvest.
Soybean production in agriculture is highly dependent on natural resources, and fertilizer application and irrigation can have a significant impact on the environment. It is important to minimize energy efficiency and environmental impact by strictly managing inputs.
Hexane (Hexane)
Hexane is produced primarily by distillation and refining from crude oil. Since hexane is a light hydrocarbon component and is obtained as a byproduct of petroleum refining, the majority of the process relies on separation and refining in distillation columns.
- energy input
- Power input: 0.5 to 1.0 kWh/kg
(used for pumps, compressors, temperature control) - Input Fuel: Fuel
- Type: Natural gas or fuel oil
- Volume: 0.15-0.25 kg/kg
(used for heating process in distillation columns)
- CO₂ emissions other than combustion
- CO₂ emissions: 0.02-0.05 kg/kg
(from by-products and volatile organic compounds in the distillation process)
- raw material input
- Name | Approximate dosage
- Crude oil | 3-5 kg (raw material including hexane component)
- process solvents and auxiliaries
- Name | Usage | Circulation Rate
- Cooling water | 6-8 kg | 90-95% (for cooling the distillation process)
- Chemicals for desulfurization (e.g., sodium hydroxide) | 0.05-0.1 kg | 80-90% recycled
- amount and type of waste disposed
- Effluent | 0.2-0.3 kg/kg (effluent from desulfurization process)
- Waste gas | 0.1-0.15 kg/kg (unreacted components and volatile organic compounds)
- changes in natural resources (e.g., water, soil)
- Water consumption: 5-10 kg/kg (used for cooling and cleaning processes)
- Soil and ore impact: None (no direct impact)
Manufacturing Process Overview
- Crude oil distillation: Crude oil is separated in an atmospheric distillation column to extract light hydrocarbon components (naphtha).
- Refining: Naphtha is further distilled to obtain a fraction containing hexane.
- Desulfurization and purification: Impurities are removed and the purified hexane is recovered as a product.
Points to keep in mind when manufacturing
- VOC control: Hexane is highly volatile, so emissions of volatile organic compounds (VOCs) must be controlled.
- Energy efficiency: Due to the high energy consumption of the distillation process, efficiency must be improved through the use of heat exchange systems.
- Liquid waste treatment: Liquid waste generated in the desulfurization process is neutralized before treatment.
Hexane production requires a lot of energy for distillation and purification, so efficient use of fuel and cooling water is critical. Because it is part of the petroleum refining process, proper management of waste is required.
Vegetable oil degreaser
The main methods for processing defatted vegetable oil (residue) are solvent extraction method of defatted oil and refining/recycling of oil cake. In general, oil removal is performed using solvents such as hexane. The following is a guideline for processing 1 kg of defatted material.
- energy input
- Power input: 0.3-0.6 kWh/kg
(used for stirring, pumps in the extraction process, and temperature control) - Input Fuel: Fuel
- Type: Natural Gas
- Quantity: 0.1-0.2 kg/kg
(used for solvent evaporation and recovery process)
- CO₂ emissions other than combustion
- CO₂ emissions: 0.01-0.03 kg/kg
(emissions from use and decomposition of organic solvents)
- raw material input
- Name | Approximate amount of dosage
- Oil cake (defatted) | 1.0 kg
- Water | 0.5 to 1.0 kg (for cleaning process)
- process solvents and auxiliaries
- Name | Usage | Circulation Rate
- Hexane | 0.3-0.5 kg | 95-98% (reused in the extraction process)
- Cooling water | 3-5 kg | 90-95% (for cooling and solvent recovery)
- amount and type of waste disposed
- Effluent | 0.2-0.3 kg/kg (residual solvent or cleaning solution)
- Solid waste | 0.1-0.2 kg/kg (fibrous residue, can be reused)
- changes in natural resources (e.g., water, soil)
- Water consumption: 4-6 kg/kg (used for washing and cooling processes)
- Soil and ore impact: None (not used directly in the manufacturing process)
Manufacturing Process Overview
- Solvent extraction: Removes residual oil from defatted material using a solvent such as hexane.
- Solvent recovery: Used solvents are evaporated and recovered for reuse.
- Waste Disposal: After extraction, solid residues are often reused as feed or fertilizer.
Points to keep in mind when manufacturing
- Solvent management: use closed systems and recovery equipment to minimize VOC (volatile organic compound) emissions.
- Increased energy efficiency: Utilize heat exchange systems to efficiently recover evaporated solvents.
- Reuse of residues: After processing, solids can be reused as feed or fertilizer, minimizing waste.
In this process, solvent reuse is key to reducing costs and environmental impact. In addition, the effective use of residues reduces the amount of waste after treatment.
xanthogenic acid ester
Xanthogenates are typically produced in reactions from alcohol and carbon disulfide (CS₂) and are used primarily as mineral flotation agents. Below is a rough estimate of inputs and energy for a typical production process.
- energy input
- Power input: 0.5-0.8 kWh/kg
(used for reactor agitation, temperature control, and pump operation) - Input Fuel: Fuel
- Type: Natural gas or fuel oil
- Volume: 0.1 to 0.15 kg/kg
(for heating and drying processes)
- CO₂ emissions other than combustion
- CO₂ emissions: 0.02-0.05 kg/kg
(trace CO₂ from reactions and waste)
- raw material input
- Name | Approximate amount of dosage
- Alcohol (methanol/ethanol) | 0.4-0.5 kg
- Carbon disulfide (CS₂) | 0.5-0.6 kg
- Caustic soda (NaOH) | 0.1 to 0.15 kg
- Water | 0.3 to 0.5 kg
- process solvents and auxiliaries
- Name | Amount Used | Circulation Rate
- Cooling water | 4-6 kg | 90-95% (for reaction cooling)
- Acetone | 0.1-0.2 kg | 85-90% (used for cleaning)
- amount and type of waste disposed
- Effluent | 0.3-0.5 kg/kg (unreacted material and washing solution)
- Gas waste | 0.1-0.15 kg/kg (CS₂ vapor and volatile components)
- Volatile gases are reused or detoxified in a recovery system.
- changes in natural resources (e.g., water, soil)
- Water consumption: 5-8 kg/kg (used for cooling and cleaning processes)
- Soil and ore impact: None (no direct impact on manufacturing process)
Manufacturing Process Overview
- Reaction of alcohol with CS₂: Alcohol and carbon disulfide react in the presence of caustic soda to produce xanthogenic acid ester.
- Washing and separation: Wash the product and separate unreacted material.
- Drying and Concentrating: Drying the final product to improve purity.
Points to keep in mind when manufacturing
- Control of CS₂: Carbon disulfide is toxic and requires strict emission control and recovery.
- Increased energy efficiency: Utilize heat exchangers to optimize energy efficiency of cooling water and heating processes.
- Reuse of waste: Collected unreacted materials and solvents can be reused to reduce costs and environmental impact.
Carbon disulfide control and reduction of volatile organic compound (VOC) emissions are important in this process. The recirculation of cooling water and solvents improves energy efficiency and environmental performance.
Detergent for separation membranes
Detergents for separation membranes consist of a mixture of surfactants, chelating agents, and acid/alkali components intended to remove membrane contaminants. Production includes processes such as mixing and agitation of the components, drying, and purification.
- energy input
- Power input: 0.6-1.0 kWh/kg
(used for agitators, temperature control, and transfer pumps) - Input Fuel: Fuel
- Type: Natural gas or fuel oil
- Volume: 0.05-0.1 kg/kg
(used for heating in the drying process)
- CO₂ emissions other than combustion
- CO₂ emissions: 0.01-0.03 kg/kg
(byproducts of chemical reactions and manufacturing processes)
- raw material input
- Name | Approximate amount of dosage
- Surfactant (sodium lauryl sulfate, etc.) | 0.3-0.5 kg
- Chelating agent (EDTA, etc.) | 0.1-0.2 kg
- Acids (e.g. phosphoric acid) | 0.05-0.1 kg
- Alkali (sodium hydroxide) | 0.1-0.2 kg
- Water | 0.2 to 0.4 kg
- process solvents and auxiliaries
- Name | Usage | Circulation Rate
- Cooling water | 4-6 kg | 90-95% (for reaction cooling)
- Ethanol | 0.1-0.2 kg | 85-90% (for cleaning process)
- amount and type of waste disposed
- Effluent | 0.3-0.4 kg/kg (cleaning water and unused components)
- Solid waste | 0.05-0.1 kg/kg (filtration residue and impurities)
- changes in natural resources (e.g., water, soil)
- Water consumption: 5-8 kg/kg (used for cooling and cleaning processes)
- Soil and ore impact: None (not used directly in the manufacturing process)
Manufacturing Process Overview
- Raw material formulation: Mix surfactants, chelating agents, acids and alkalis in appropriate proportions.
- Stirring and reaction: Stirring and temperature control are used to homogenize the solution.
- Drying and Concentrating: Heating and concentrating as needed to obtain a liquid or powdered product.
- Waste Disposal: Properly dispose of waste liquids and impurities from the cleaning process.
Points to keep in mind when manufacturing
- VOC control: When organic solvents such as ethanol are used, VOC emission control is required.
- Increased energy efficiency: Use heat exchange systems to use cooling and heating energy more efficiently.
- Waste minimization: Waste volume is reduced by reusing liquid waste and recovering unreacted components.
In the production of detergents for separation membranes, control of chemical reactions and uniform mixing of components are the key to quality control. In addition, the environmental impact must be minimized through the reuse of waste liquids and solvents.
Concentrated sludge treatment
Dewatering, drying, incineration, and composting are the industrial processes used to treat concentrated sludge. Here are input and energy guidelines based on a typical process combining dewatering and incineration.
- energy input
- Power input: 0.3-0.5 kWh/kg
(used for dehydrator, agitator, pump operation, air compressor) - Input Fuel: Fuel
- Type: Natural gas or fuel oil
- Quantity: 0.05-0.1 kg/kg
(used for drying and incineration processes)
- CO₂ emissions other than combustion
- CO₂ emissions: 0.01-0.03 kg/kg
(emissions from sludge decomposition and chemical reactions)
- raw material input
- Name | Approximate amount of dosage
- Concentrated sludge | 1.0 kg
- Polymer flocculant | 0.005 to 0.01 kg
- process solvents and auxiliaries
- Name | Usage | Circulation Rate
- Cooling water | 2-3 kg | 90-95% (cooling process at incineration facility)
- Flocculation aid | 0.01-0.02 kg | 85-90% (for dewatering process)
- amount and type of waste disposed
- Ash content | 0.1-0.2 kg/kg (incineration residue)
- Exhaust gas | 0.02-0.05 kg/kg (treated by gas treatment equipment)
- Wastewater | 0.2-0.3 kg/kg (wastewater from dewatering process)
- changes in natural resources (e.g., water, soil)
- Water consumption: 3-5 kg/kg (used for cooling and cleaning processes)
- Soil and ore impact: Incinerated ash can be landfilled or reused for construction materials
Manufacturing Process Overview
- Dewatering: Dewater sludge by adding polymer flocculants to reduce water content.
- Drying and Incineration: After dehydration, the sludge is incinerated to break down the organic components into ash.
- Exhaust gas treatment: Exhaust gas generated during incineration is treated by filtration and adsorption equipment.
- Wastewater treatment: Wastewater from the dewatering process is purified.
Points to keep in mind when manufacturing
- Exhaust gas control: A gas treatment system during incineration removes hazardous substances such as dioxins.
- Ash Reuse: Incinerated ash can be used for landfill as well as cement material.
- Increased energy efficiency: Heat exchange systems reuse incineration heat and reduce energy consumption.
Energy efficiency and environmental management are critical in this process. Proper treatment of wastewater and flue gas and reuse of incinerated ash minimize the environmental impact.
propylene
Propylene is produced by naphtha cracking and propane dehydrogenation. Below is a rough estimate of energy and resource inputs, primarily based on the naphtha cracking method.
- energy input
- Power input: 0.5-0.8 kWh/kg
(used for compressor, agitation, and temperature control) - Input Fuel: Fuel
- Type: Natural gas or fuel oil
- Quantity: 0.6-0.8 kg/kg
(used for heating naphtha cracking furnaces)
- CO₂ emissions other than combustion
- CO₂ emissions: 0.05-0.1 kg/kg
(from byproduct treatment and unburned hydrocarbons)
- raw material input
- Name | Approximate amount of dosage
- Naphtha | 2.0 to 2.5 kg
- Water vapor | 0.4 to 0.6 kg (for cracking)
- process solvents and auxiliaries
- Name | Usage | Circulation Rate
- Cooling water | 10-15 kg | 90-95% (for heat exchange)
- Desulfurizer (e.g., zinc oxide) | 0.01-0.02 kg | Recyclable
- amount and type of waste disposed
- Waste gas | 0.1 to 0.15 kg/kg (unreacted gas and byproducts)
- Effluent | 0.02-0.05 kg/kg (treated FGD effluent and cooling water)
- changes in natural resources (e.g., water, soil)
- Water consumption: 10-20 kg/kg (for cooling and steam generation)
- Soil and ore impact: None (little direct impact on manufacturing process)
Manufacturing Process Overview
- Naphtha cracking: Naphtha is heated at high temperature and cracked with steam to produce light hydrocarbons including propylene.
- Separation and purification: The product is cooled to separate the gas and liquid components and purify the propylene to a high purity.
- Byproduct treatment: Byproducts such as ethylene, butadiene, and other compounds are also recovered as products.
Points to keep in mind when manufacturing
- Control of high temperature reactions: Control of temperature and pressure is important to prevent side reactions due to overheating.
- Waste recovery and reuse: By-products are reused as raw materials for other chemical products.
- Increased energy efficiency: The use of a heat exchange system improves the energy efficiency of the cracking process.
The process is energy intensive, so heat recovery and reuse of byproducts are key to reducing costs and environmental impact.
Hydrogen chloride (HCl)
Gas absorption, incineration, or neutralization are typically used industrially to treat hydrogen chloride. Here is a guide to the gas absorption method (absorption with water) and the subsequent process involving a neutralization step.
- energy input
- Power input: 0.3-0.6 kWh/kg
(operation of pumps, agitators, and temperature controllers) - Input Fuel: Fuel
- Type: Natural gas or fuel oil
- Quantity: 0.05-0.1 kg/kg
(used for gas re-burning process if necessary)
- CO₂ emissions other than combustion
- CO₂ emissions: 0.02-0.05 kg/kg
(from neutralization reaction and unreacted gas)
- raw material input
- Name | Approximate amount of dosage
- Hydrogen chloride (HCl) | 1.0 kg
- Water | 1.0-1.5 kg (for absorption)
- Sodium hydroxide (NaOH) | 0.4-0.5 kg (for neutralization)
- process solvents and auxiliaries
- Name | Usage | Circulation Rate
- Cooling water | 5-8 kg | 90-95% (for cooling the absorption column)
- Cleaning water | 0.5-1.0 kg | 85-90% (equipment cleaning)
- amount and type of waste disposed
- Brine effluent | 1.2 to 1.5 kg/kg (HCl and NaOH neutralized)
- Waste gas | 0.05-0.1 kg/kg (unreacted components and water vapor)
- changes in natural resources (e.g., water, soil)
- Water consumption: 7-10 kg/kg (cooling, absorption, and cleaning processes)
- Soil and ore impact: None (no direct impact on manufacturing process)
Overview of the treatment process
- Absorption: Hydrogen chloride gas is absorbed by water to form hydrochloric acid (HCl solution).
- Neutralization: The absorbed hydrochloric acid is neutralized with sodium hydroxide (NaOH) to produce brine.
- Waste Disposal: After neutralization, the brine is properly treated or reused. Unreacted gases are treated in a filtering system.
Points to keep in mind when manufacturing
- Exhaust gas control: Increases HCl gas absorption efficiency and minimizes unreacted gases.
- Energy Efficiency: Utilize cooling water circulation systems to reduce energy use.
- Reuse of effluent: After neutralization, the brine can be reused in industrial applications or disposed of safely.
The key to this process is the complete absorption and neutralization of hydrogen chloride gas. The process must be operated with minimal environmental impact through energy efficiency and proper management of flue gas and wastewater.
Nitrogen fertilizer (e.g., urea fertilizer)
Nitrogen fertilizers are produced primarily from ammonia synthesis and subsequent reactions. Here are the inputs and energy guidelines based on the production of a typical nitrogen fertilizer, **urea (CO(NH₂)₂)**.
- energy input
- Power input: 0.6-1.0 kWh/kg
(operation of compressors, agitators, pumps, and temperature controllers) - Input Fuel: Fuel
- Type: Natural gas (main raw material and fuel for ammonia production)
- Quantity: 0.7 to 1.0 kg/kg
(used for reactor heating and hydrogen production)
- CO₂ emissions other than combustion
- CO₂ emissions: 0.3-0.5 kg/kg
(byproduct of natural gas reforming and ammonia synthesis processes)
- raw material input
- Name | Approximate amount of dosage
- Ammonia (NH₃) | 0.6-0.7 kg
- Carbon dioxide (CO₂) | 0.3-0.4 kg
(as raw material for urea synthesis)
- process solvents and auxiliaries
- Name | Usage | Circulation Rate
- Cooling water | 10-15 kg | 90-95% (removal of reaction heat)
- Steam | 0.5-1.0 kg | 80-90% (reaction acceleration and temperature maintenance)
- amount and type of waste disposed
- Effluent | 0.1-0.2 kg/kg (cleaning water and impurities)
- Exhaust gas | 0.05-0.1 kg/kg (unreacted gas and byproducts)
- changes in natural resources (e.g., water, soil)
- Water consumption: 5-8 kg/kg (cooling and cleaning processes)
- Soil and ore impact: None (little direct impact on manufacturing process)
Manufacturing Process Overview
- Ammonia production: Natural gas is reacted with water vapor to produce hydrogen, which is then synthesized with nitrogen to produce ammonia.
- Urea Synthesis: Reaction of ammonia and carbon dioxide to produce urea.
- Cooling and crystallization: Cools the urea solution and crystallizes and dries the solid urea.
- Waste Disposal: Properly dispose of unreacted gas and liquid waste.
Points to keep in mind when manufacturing
- Increased energy efficiency: Waste heat from the reaction is recovered and reused in the steam and cooling system.
- Effective use of CO₂: CO₂ byproducts from ammonia production are utilized in urea synthesis to reduce emissions.
- Exhaust gas control: Unreacted gases generated in the manufacturing process are recovered and reused.
Because of the high energy consumption of this process, the reuse of waste heat and the effective use of CO₂ are key to reducing environmental impact and costs.
phosphate fertilizer
Phosphate fertilizers (e.g., ammonium dihydrogen phosphate and tricalcium phosphate) are produced by processing phosphate ores or by reaction with sulfuric acid. The following are guidelines based on sulfuric acid treatment methods using typical phosphate ores.
- energy input
- Power input: 0.3-0.5 kWh/kg
(crusher, agitator, pump operation) - Input Fuel: Fuel
- Type: Natural gas or fuel oil
- Quantity: 0.05 to 0.1 kg/kg
(for heating and drying processes in reactors)
- CO₂ emissions other than combustion
- CO₂ emissions: 0.05-0.1 kg/kg
(emissions from decomposition and sulfuric acid reaction from phosphate ore)
- raw material input
- Name | Approximate amount of dosage
- Phosphorus ore (Ca₃(PO₄)₂) | 1.2-1.5 kg
- Sulfuric acid (H₂SO₄) | 0.6-0.8 kg
- Ammonia (NH₃) | 0.2 to 0.3 kg (for ammonium phosphate)
- process solvents and auxiliaries
- Name | Usage | Circulation Rate
- Cooling water | 5-7 kg | 90-95% (for reaction cooling)
- Cleaning water | 0.5-1.0 kg | 85-90% (for equipment cleaning)
- amount and type of waste disposed
- Effluent | 0.1-0.2 kg/kg (unused portion of sulfuric acid and washing water)
- Gypsum waste (CaSO₄) | 0.5-0.6 kg/kg (generated as byproduct)
- changes in natural resources (e.g., water, soil)
- Water consumption: 6-10 kg/kg (for cooling and cleaning)
- Soil and ore impacts: mining phosphate ore requires soil excavation
Manufacturing Process Overview
- Phosphate crushing: Phosphate ore is crushed to make it more reactive with sulfuric acid.
- Reaction with sulfuric acid: Adding sulfuric acid produces phosphoric acid and gypsum.
- Reaction with ammonia (if applicable): Reaction of phosphoric acid with ammonia to form ammonium phosphate.
- Drying and Cooling: The final product is dried, cooled, and packaged.
- Waste Disposal: Properly dispose of or recycle gypsum by-products.
Points to keep in mind when manufacturing
- Byproduct management: Gypsum is often reused as a construction material, but management during disposal is also important.
- Waste gas and effluent treatment: Proper treatment of gases and effluents generated during reactions reduces environmental impact.
- Water and energy efficiency: Reduced use of water resources through recycling of cooling and cleaning water.
In this process, the reuse of gypsum and proper treatment of liquid waste are important to reduce environmental impact. In addition, the recycling of cooling water and energy is expected to reduce production costs.
potash fertilizer
Potassium fertilizers are produced primarily by extraction from mineral resources in salt lakes (e.g., carnallite) or by chemical reactions with potassium sulfate. Below are the inputs and energy guidelines based on the production process, which is primarily based on **potassium chloride (KCl)**.
- energy input
- Power input: 0.4-0.7 kWh/kg
(crushing, beneficiation, drying, agitator operation) - Input Fuel: Fuel
- Type: Natural gas or fuel oil
- Quantity: 0.05-0.1 kg/kg
(used in drying and heat treatment processes)
- CO₂ emissions other than combustion
- CO₂ emissions: 0.02-0.05 kg/kg
(from mineral processing and reaction byproducts)
- raw material input
- Name | Approximate amount of dosage
- Carnalite (KCl, MgCl₂, 6H₂O) | 1.5 to 2.0 kg
- Water | 1.0-1.5 kg (for extraction)
- process solvents and auxiliaries
- Name | Usage | Circulation Rate
- Cooling water | 4-6 kg | 90-95% (removal of reaction heat)
- Cleaning water | 0.5-1.0 kg | 85-90% (equipment cleaning)
- amount and type of waste disposed
- Salt Effluent | 0.2-0.3 kg/kg (unused salt and byproducts)
- Solid waste | 0.05-0.1 kg/kg (impurities and residues)
- changes in natural resources (e.g., water, soil)
- Water consumption: 5-8 kg/kg (cooling and extraction processes)
- Soil and ore impacts: Mineral mining affects soils in mined areas
Manufacturing Process Overview
- Mineral extraction: Mineral ores such as carnallite are mined and dissolved in water to extract KCl.
- Beneficiation and Separation: Separates salts and extracts pure KCl.
- Drying and Purification: The final product is dried and purified to a powder or crystallized form.
- Waste Disposal: Properly dispose of by-product effluents and residues.
Points to keep in mind when manufacturing
- Waste Management: Proper disposal of effluent and salt waste is important. Reuse of waste materials reduces environmental impact.
- Increased energy efficiency: Heat exchange systems reuse energy from the drying process.
- Water recycling: Maximize reuse of water used for extraction and cooling.
In this process, the reuse of salts and the recycling of water are important to reduce the environmental impact. Environmental management of the mining site is also essential for sustainable operation.
corn seed
Corn seed is obtained through the process of growing, harvesting, drying, and sorting. Below is a rough estimate of inputs and energy for commercial-scale seed production.
- energy input
- Power input: 0.2-0.4 kWh/kg
(for drying, sorting, and conveying equipment) - Input Fuel: Fuel
- Type: Diesel oil or biofuel
- Quantity: 0.3-0.5 kg/kg
(cultivating, harvesting, and transporting farm machinery operations)
- CO₂ emissions other than combustion
- CO₂ emissions: 0.01-0.03 kg/kg
(from fertilizer use and soil microbial activity)
- raw material input
- Name | Approximate amount of dosage
- Seed | 0.01-0.02 kg (mother seed)
- Nitrogen fertilizer (N) | 0.05-0.1 kg
- Phosphate fertilizer (P₂O₅) | 0.03-0.05 kg
- Potash fertilizer (K₂O) | 0.02-0.04 kg
- Irrigation water | 5-7 kg
- process solvents and auxiliaries
- Name | Usage | Circulation Rate
- Cooling water | 3-5 kg | 90-95% (cooling of drying process)
- Pesticides (insecticides and herbicides) | 0.005-0.01 kg | Decompose after use or released into the environment
- amount and type of waste disposed
- Plant residues (stems and leaves) | 0.5-1.0 kg/kg (reused as feed or compost)
- Waste pesticide containers | 0.001~0.002 kg/kg (properly collected and disposed)
- changes in natural resources (e.g., water, soil)
- Water consumption: 5,000-7,000 L/kg (irrigation and cooling processes)
- Soil and ore effects: soil variability due to cultivation and fertilizer application has an effect.
Manufacturing Process Overview
- Tilling and seeding: Till the soil and sow the mother seed.
- Fertilizer application and irrigation: Nourishes and promotes growth.
- Pesticide Management: Appropriate pesticides are applied to prevent pests and diseases.
- Harvesting and Drying: After harvesting, the seeds are dried, sorted and refined.
Points to keep in mind when manufacturing
- Pesticide Management: Reduces environmental impact by preventing overspray.
- Waste Reuse: Plant residues can be reused as feed or compost to reduce waste.
- Optimize water use: Optimize irrigation systems for more efficient use of water resources.
Water and energy use efficiency is critical in this process. Waste is reduced through the reuse of plant residues, and environmental impact is minimized through proper management of pesticides and fertilizers.
Herbicide
Herbicides are manufactured through organic chemical reactions and typical products include glyphosate and 2,4-D. Below are some inputs and energy estimates for typical herbicide production.
- energy input
- Power input: 0.5-1.0 kWh/kg
(agitator, temperature controller, pump operation) - Input Fuel: Fuel
- Type: Natural gas or fuel oil
- Volume: 0.1-0.2 kg/kg
(used for heating and drying processes)
- CO₂ emissions other than combustion
- CO₂ emissions: 0.02-0.05 kg/kg
(emissions from chemical reactions and solvent evaporation)
- raw material input
- Name | Approximate amount of dosage
- Glycine (or chloroacetic acid, etc.) | 0.3-0.5 kg
- Phosphonic acid (or phosphate) | 0.2-0.3 kg
- Sodium hydroxide (NaOH) | 0.1 to 0.15 kg
- Water | 0.2 to 0.5 kg
- process solvents and auxiliaries
- Name | Usage | Circulation Rate
- Acetone | 0.1-0.2 kg | 85-95% (used for cleaning)
- Cooling water | 5-8 kg | 90-95% (reaction heat removal)
- amount and type of waste disposed
- Effluent | 0.2-0.3 kg/kg (unreacted material and washing solution)
- Organic waste | 0.05-0.1 kg/kg (byproduct residue)
- changes in natural resources (e.g., water, soil)
- Water consumption: 6-10 kg/kg (used for cooling and cleaning processes)
- Soil and ore impact: None (not used directly in the manufacturing process)
Manufacturing Process Overview
- Reaction: The main component of the herbicide is synthesized by reacting glycine or chloroacetic acid with phosphonic acid.
- Neutralization: Neutralize with NaOH to balance acidity and basicity.
- Purification and drying: The product is purified and the solvent is evaporated to obtain a solid or liquid product.
- Waste Disposal: Properly dispose of byproducts and unused solvents.
Points to keep in mind when manufacturing
- VOC emission control: A sealing system is required to minimize emissions of volatile organic compounds (e.g., acetone).
- Increased energy efficiency: save energy by utilizing cooling water and waste heat recovery systems.
- Reuse of waste: Some of the byproducts generated can be reused in other chemical processes.
In this process, solvent reuse and proper disposal of waste are key to reducing environmental impact. In addition, a heat exchange system is installed to increase energy efficiency.
Insecticides
Insecticides are manufactured through organic chemical synthesis, and typical examples include pyrethroids and neonicotinoids. The following is a guide based on the manufacture of pyrethroid insecticides.
- energy input
- Power input: 0.5 to 1.0 kWh/kg
(agitation, temperature control, pumps, refinery operation) - Input Fuel: Fuel
- Type: Natural gas or fuel oil
- Volume: 0.1-0.2 kg/kg
(used for heating, drying, and solvent evaporation processes)
- CO₂ emissions other than combustion
- CO₂ emissions: 0.02-0.05 kg/kg
(emissions from chemical reactions and solvent evaporation)
- raw material input
- Name | Approximate amount of dosage
- Cyanopyrithrin (intermediate) | 0.3-0.5 kg
- Ethanol | 0.1 to 0.2 kg
- Acetic acid (catalyst) | 0.05-0.1 kg
- Sodium hydroxide (NaOH) | 0.05-0.1 kg
- Water | 0.2 to 0.4 kg
- process solvents and auxiliaries
- Name | Usage | Circulation Rate
- Acetone | 0.2-0.3 kg | 90-95% (used for cleaning and extraction processes)
- Cooling water | 5-8 kg | 90-95% (used for temperature control and refining process)
- amount and type of waste disposed
- Effluent | 0.2-0.4 kg/kg (washing liquid and unreacted materials)
- Organic waste | 0.05-0.1 kg/kg (byproduct residue)
- changes in natural resources (e.g., water, soil)
- Water consumption: 6-10 kg/kg (used for cooling and cleaning)
- Soil and ore effects: None (not directly affected by the manufacturing process)
Manufacturing Process Overview
- Chemical synthesis: Cyanopyrithrin and other intermediates react in the presence of ethanol and acetic acid.
- Neutralize and clean: Neutralize with sodium hydroxide to remove residual acid.
- Purification and drying: The product is extracted with a solvent such as acetone and dried.
- Waste Disposal: Properly dispose of byproducts and unused solvents.
Points to keep in mind when manufacturing
- Control of VOC emissions: Use closed systems and recovery equipment to minimize emissions of organic solvents such as acetone.
- Increased energy efficiency: utilize cooling water circulation and heat exchange systems.
- Waste minimization: Reduce waste by reusing byproducts and recovering unreacted materials.
In this process, solvent reuse and proper disposal of waste liquids are key to reducing environmental impact. Efficient use of cooling water and energy reuse are key to sustainable operation.
glucose
Industrially, glucose is produced primarily by hydrolysis (enzymatic or acid treatment) of starch. Below are the inputs and energy requirements for glucose production using enzymatic methods.
- energy input
- Power input: 0.4-0.7 kWh/kg
(used for agitation, heating, and pump operation) - Input Fuel: Fuel
- Type: Natural gas or fuel oil
- Quantity: 0.05-0.1 kg/kg
(used for drying and heating processes)
- CO₂ emissions other than combustion
- CO₂ emissions: 0.01-0.03 kg/kg
(from enzymatic reactions and byproducts)
- raw material input
- Name | Approximate dosage
- Starch (corn, etc.) | 1.1-1.3 kg
- Water | 2 to 3 kg
- Enzymes (amylase, glucoamylase) | 0.01-0.02 kg
- process solvents and auxiliaries
- Name | Usage | Circulation Rate
- Cooling water | 5-8 kg | 90-95% (reaction temperature control)
- Acetone | 0.05-0.1 kg | 85-90% (for cleaning process)
- amount and type of waste disposed
- Effluent | 0.2-0.4 kg/kg (unused water and washing water)
- Solid waste | 0.1-0.2 kg/kg (starch residue and enzyme residue)
- changes in natural resources (e.g., water, soil)
- Water consumption: 5-10 kg/kg (used for cooling and cleaning)
- Soil and mineral impacts: indirect (cultivation of corn and other raw material plants)
Manufacturing Process Overview
- Hydrolysis: Starch is mixed with water and enzymes are added to break it down into glucose.
- Heating and stirring: Temperature and stirring are controlled to ensure that the reaction proceeds efficiently.
- Purification and Concentration: The glucose solution produced is purified and concentrated to a liquid or powder form.
- Drying: If necessary, glucose is dried to a powder form.
- Waste Disposal: Properly dispose of byproducts and unused solutions.
Points to keep in mind when manufacturing
- Temperature and pH control: Proper temperature and pH are important to optimize enzyme activity.
- Reuse of waste: Starch residues and effluents can be reused in other manufacturing processes.
- Increased energy efficiency: Reduces energy consumption by reusing cooling water and waste heat.
In this process, optimal use of enzymes and recycling of water and energy are important to reduce costs and environmental impact. Environmental impacts from the cultivation of raw materials such as corn are also considered.
ammonium salt
Ammonium salts are produced by the reaction of ammonia with acids (sulfuric acid, hydrochloric acid, etc.). Below is an input and energy guideline using ammonium sulfate as an example.
- energy input
- Power input: 0.5-0.8 kWh/kg
(agitation, temperature control, pump operation) - Input Fuel: Fuel
- Type: Natural gas or fuel oil
- Volume: 0.1 to 0.15 kg/kg
(used for drying and maintaining reaction temperature)
- CO₂ emissions other than combustion
- CO₂ emissions: 0.02-0.04 kg/kg
(ammonia production and byproducts from the process)
- raw material input
- Name | Approximate amount of dosage
- Ammonia (NH₃) | 0.2-0.3 kg
- Sulfuric acid (H₂SO₄) | 0.7-0.8 kg
- process solvents and auxiliaries
- Name | Usage | Circulation Rate
- Cooling water | 5-7 kg | 90-95% (used to control reaction temperature)
- Cleaning water | 0.2-0.5 kg | 85-90% (equipment cleaning)
- amount and type of waste disposed
- Effluent | 0.1-0.2 kg/kg (unreacted material and washing water)
- Solid waste | 0.05-0.1 kg/kg (impurities and residues)
- changes in natural resources (e.g., water, soil)
- Water consumption: 5-8 kg/kg (used for cooling and cleaning)
- Soil and ore impact: None (not used directly in the manufacturing process)
Manufacturing Process Overview
- Reaction: Ammonia reacts with sulfuric acid to produce ammonium sulfate.
- Cooling and crystallization: After the reaction, the solution is cooled and the ammonium sulfate is crystallized.
- Drying and grinding: Crystals are dried and ground into a powder.
- Waste Disposal: Properly dispose of unused acid and cleaning waste.
Points to keep in mind when manufacturing
- Increased energy efficiency: use cooling water circulation system to recycle waste heat.
- Waste Reduction: Recovery of unreacted materials and reuse of byproducts leads to cost savings and reduced environmental impact.
- VOC control: Prevent leakage of ammonia gas and collect it safely.
In this process, reuse of cooling water and recovery of unused components are important to reduce environmental impact and costs. In addition, temperature control during production and prevention of gas leakage are key to safe operation.
Alkali catalysts (e.g., sodium hydroxide, potassium methylate)
Alkali catalysts are produced by the reaction of alkali hydroxide or with alcohols. Here are some inputs and energy estimates based on the production of typical **sodium hydroxide (NaOH) and potassium methylate (CH₃OK)**.
- energy input
- Power input: 0.5-1.0 kWh/kg
(used for agitation, electrolyzer, temperature control, and conveying equipment) - Input Fuel: Fuel
- Type: Natural gas or fuel oil
- Quantity: 0.05-0.1 kg/kg
(used in drying and heat treatment processes)
- CO₂ emissions other than combustion
- CO₂ emissions: 0.01-0.03 kg/kg
(from alkali reactions and byproducts)
- raw material input
- Name | Approximate amount of dosage
- Sodium chloride (NaCl) | 2.0 to 2.5 kg (for electrolytic method)
- Methanol (CH₃OH) | 0.3 to 0.5 kg (for potassium methylate)
- Potassium (K) or sodium (Na) | 0.5-0.7 kg
- process solvents and auxiliaries
- Name | Usage | Circulation Rate
- Cooling water | 4-8 kg | 90-95% (for electrolysis and reaction temperature control)
- Cleaning water | 0.5-1.0 kg | 85-90% (for equipment cleaning)
- amount and type of waste disposed
- Effluent | 0.2-0.3 kg/kg (unreacted material and washing effluent)
- Solid waste | 0.05-0.1 kg/kg (electrolysis byproducts and residues)
- changes in natural resources (e.g., water, soil)
- Water consumption: 6-10 kg/kg (used for cooling and cleaning processes)
- Soil and ore impact: None (manufacturing process has no impact on soil)
Manufacturing Process Overview
- Raw material reaction: Sodium hydroxide is produced from sodium chloride by electrolysis. For potassium methylate, potassium reacts with methanol.
- Purification and drying: The product is filtered, purified, and dried.
- Cooling and crystallization: Lower the temperature and allow the product to crystallize or formulate into a liquid formulation.
- Waste Disposal: Properly dispose of liquid waste and unreacted materials.
Points to keep in mind when manufacturing
- Optimization of the electrolysis process: use efficient electrolysis systems to reduce power consumption.
- VOC control: Use closed systems and recovery equipment to reduce emissions of volatile substances such as methanol.
- Cooling water reuse: Circulates cooling water to improve energy efficiency.
Reduced power consumption and waste management are important in this process. Reuse of cooling water and waste liquids is expected to reduce costs and environmental impact.
Poly aluminum chloride (PAC)
Polyaluminum chloride (PAC) is produced by reacting aluminum hydroxide with hydrochloric acid and is widely used as a water treatment agent in industry. Below are inputs and energy guidelines based on a typical manufacturing process.
- energy input
- Power input: 0.3-0.5 kWh/kg
(agitation, reaction temperature control, pump operation) - Input Fuel: Fuel
- Type: Natural gas or fuel oil
- Volume: 0.05-0.1 kg/kg
(used for heating and drying processes)
- CO₂ emissions other than combustion
- CO₂ emissions: 0.01-0.03 kg/kg
(byproduct of reaction between hydrochloric acid and aluminum hydroxide)
- raw material input
- Name | Approximate amount of dosage
- Aluminum hydroxide (Al(OH)₃) | 0.35-0.45 kg
- Hydrochloric acid (HCl) | 0.55 to 0.65 kg
- Water | 0.5 to 0.8 kg (for dissolution and reaction)
- process solvents and auxiliaries
- Name | Usage | Circulation Rate
- Cooling water | 5-7 kg | 90-95% (reaction temperature control)
- Cleaning water | 0.2-0.5 kg | 85-90% (for equipment cleaning)
- amount and type of waste disposed
- Effluent | 0.1-0.2 kg/kg (unreacted material and washing effluent)
- Solid waste | 0.05-0.1 kg/kg (impurities and residues)
- changes in natural resources (e.g., water, soil)
- Water consumption: 6-9 kg/kg (used for cooling and cleaning processes)
- Soil and ore impact: None (no direct impact on soil during manufacturing process)
Manufacturing Process Overview
- Dissolution and reaction: Dissolve aluminum hydroxide in hydrochloric acid to produce polyaluminum chloride (PAC).
- Agitation and temperature control: Agitation and temperature control are used to maintain a uniform reaction.
- Purification and Concentration: The product is filtered to remove impurities and then concentrated as needed.
- Waste disposal: Properly dispose of byproducts and unreacted materials.
Points to keep in mind when manufacturing
- Waste management: Generated liquid and solid wastes are properly disposed of to reduce environmental impact.
- Cooling Water Reuse: Improves energy efficiency by circulating cooling water.
- Reaction optimization: Minimize unreacted product by adjusting reaction conditions.
In this process, reduction of energy consumption and proper disposal of waste are important to reduce environmental impact. In addition, reuse of cooling water contributes to cost reduction.
Sodium hypochlorite (NaClO)
Sodium hypochlorite is produced by the reaction of chlorine gas and sodium hydroxide and is widely used as a bleaching agent and disinfectant. Below is a rough estimate of inputs and energy for industrial production.
- energy input
- Power input: 0.4-0.6 kWh/kg
(for agitator, compressor, and reaction temperature control) - Input Fuel: Fuel
- Type: Natural gas or fuel oil
- Volume: 0.05 to 0.1 kg/kg
(for heating and drying processes in reaction tanks)
- CO₂ emissions other than combustion
- CO₂ emissions: 0.01-0.03 kg/kg
(from reacted and unused components)
- raw material input
- Name | Approximate amount of dosage
- Chlorine gas (Cl₂) | 0.7 to 0.9 kg
- Sodium hydroxide (NaOH) | 0.3-0.5 kg
- Water | 0.5 to 1.0 kg
- process solvents and auxiliaries
- Name | Usage | Circulation Rate
- Cooling water | 5-8 kg | 90-95% (used to control reaction temperature)
- Cleaning water | 0.3-0.5 kg | 85-90% (for equipment cleaning)
- amount and type of waste disposed
- Effluent | 0.1-0.2 kg/kg (washing effluent and unreacted material)
- Gas waste | 0.02-0.05 kg/kg (unused chlorine and water vapor)
- changes in natural resources (e.g., water, soil)
- Water consumption: 6-10 kg/kg (for cooling and cleaning)
- Soil and ore impact: None (no impact on soil during manufacturing process)
Manufacturing Process Overview
- Chlorine and NaOH reaction: Chlorine gas is passed through a sodium hydroxide solution to produce sodium hypochlorite.
- Temperature control: Control temperatures to optimize reactions and reduce side reactions.
- Cooling and Concentration: The product is cooled to obtain a concentrated sodium hypochlorite solution.
- Waste Disposal: Properly collect and dispose of by-products and unused chlorine gas.
Points to keep in mind when manufacturing
- VOC and gas control: collects and safely disposes of unreacted chlorine gas.
- Improved energy efficiency: Circulation of cooling water and reuse of reaction heat improves energy efficiency.
- Proper treatment of effluent: The effluent should be neutralized or treated in an appropriate wastewater treatment facility.
Safe management of chlorine gas and reuse of cooling water are important in this process. Recovery of unreacted chlorine reduces environmental impact and energy consumption.
Sludge
Sludge treatment can be accomplished in multiple ways, including dewatering, incineration, and composting. Here is a guide to a typical combined dewatering and incineration process.
- energy input
- Power input: 0.3-0.5 kWh/kg
(operation of dehydrator, agitator, pumps, and gas treatment equipment) - Input Fuel: Fuel
- Type: Natural gas or fuel oil
- Quantity: 0.1-0.2 kg/kg
(used for incinerator heating and drying processes)
- CO₂ emissions other than combustion
- CO₂ emissions: 0.01-0.03 kg/kg
(from sludge decomposition and process byproducts)
- raw material input
- Name | Approximate amount of dosage
- Sludge | 1.0 kg
- Polymer flocculant | 0.005 to 0.01 kg
- process solvents and auxiliaries
- Name | Usage | Circulation Rate
- Cooling water | 5-8 kg | 90-95% (used for incinerator cooling process)
- Cleaning water | 0.3-0.5 kg | 85-90% (for equipment cleaning)
- amount and type of waste disposed
- Incinerated ash | 0.1-0.2 kg/kg (residue after incineration)
- Exhaust gas | 0.05-0.1 kg/kg (unreacted gas and dioxins)
- Treated with appropriate gas treatment equipment.
- changes in natural resources (e.g., water, soil)
- Water consumption: 5-10 kg/kg (used for cooling and cleaning)
- Soil and ore impact: Incinerator ash can be reclaimed or reused as construction material
Overview of the treatment process
- Dewatering: Adding a polymer flocculant to sludge removes water and reduces water content.
- Incineration: Incinerates sludge after dewatering to decompose organic matter.
- Exhaust gas treatment: Exhaust gas generated during incineration is treated by filtration and adsorption equipment.
- Disposal of incinerated ash: After incineration, the ash is either buried or reused as cement or other materials.
Points to keep in mind when manufacturing
- Control of VOCs and hazardous gases: Proper treatment of gases containing dioxins is required.
- Energy efficiency improvements: install heat exchange systems to reuse incineration heat.
- Reuse of waste: Reduce the amount of landfill waste by reusing incinerated ash as construction materials.
In this process, improving energy efficiency and recycling incinerator ash are important to reduce environmental impact. In addition, proper operation of the waste gas treatment system reduces emissions of hazardous substances.
Mine Waste Processing
Mine waste is processed primarily through the processes of dewatering, stabilization, calcination, and reuse. Below are approximate inputs and energy based on typical drying/solidification and reuse (e.g., as construction material) processes.
- energy input
- Power input: 0.4-0.7 kWh/kg
(crushing, agitation, pump operation, dewatering equipment operation) - Input Fuel: Fuel
- Type: Natural gas or fuel oil
- Quantity: 0.1-0.2 kg/kg
(used for drying and baking processes)
- CO₂ emissions other than combustion
- CO₂ emissions: 0.05-0.1 kg/kg
(from mineral reaction and dust processing)
- raw material input
- Name | Approximate amount of dosage
- Mine waste | 1.0 kg
- Lime (CaO) or cement | 0.05-0.1 kg
- Polymer additive | 0.005 to 0.01 kg
- process solvents and auxiliaries
- Name | Usage | Circulation Rate
- Cooling water | 4-8 kg | 90-95% (for reaction temperature control and furnace cooling)
- Cleaning water | 0.5-1.0 kg | 85-90% (for dust control and equipment cleaning)
- amount and type of waste disposed
- Solid waste | 0.1-0.2 kg/kg (residue of calcined ash and impurities)
- Exhaust gas | 0.02-0.05 kg/kg (dust and volatile organic matter)
- changes in natural resources (e.g., water, soil)
- Water consumption: 6-10 kg/kg (for cooling and dust suppression)
- Soil and ore impact: Calcined ash can be reused in landfills or as construction material
Overview of the treatment process
- Dewatering and grinding: dewater and grind waste to facilitate disposal.
- Stabilization: Stabilize waste by adding lime or cement.
- Firing and cooling: Firing, if necessary, to remove impurities, followed by cooling.
- Reuse or landfill of waste: After calcination, the ash is used as construction material or landfill material.
Points to keep in mind when manufacturing
- Dust and Exhaust Gas Control: Exhaust gas treatment equipment is required to properly dispose of dust and hazardous gases.
- Increased energy efficiency: Utilize heat exchange systems to increase energy efficiency of drying and firing processes.
- Reuse of waste: Reduce the amount of landfill waste by reusing the ash after calcination as construction material.
Dust and gas control and efficient use of energy are important in this process. Furthermore, the reuse of waste after treatment is expected to reduce environmental impact and utilize resources in a sustainable manner.
Percentage of composition to the global average of 1 kWh of electricity generated
Worldwide, electricity is generated from a combination of fossil fuels (coal, natural gas, and oil), renewable energy sources (solar, wind, and hydro), and nuclear power. Below are the approximate global average inputs and percentages of each fuel to generate 1 kWh of electricity.
- approximate fuel input (kg/kWh)
fossil fuel
- Coal: 0.25-0.30 kg/kWh
- Output: Converted with approximately 35-45% efficiency
- Percentage: 36% (global average in 2021)
- Natural gas: 0.18-0.20 kg/kWh
- Output: Approx. 50-60% efficiency (combined cycle power generation)
- Percentage: 23
- Oil: 0.02-0.03 kg/kWh
- Output: Approx. 25-35% efficiency
- Percentage: 3
Renewable Energy and Others
- Biomass: 0.45-0.55 kg/kWh
- Percentage: 3
- Nuclear: Uranium fuel pellets (approx. 0.00003 kg/kWh)
- Percentage: 10
- Renewable energy (wind, solar, hydro):
- Input: None (direct resource use)
- Percentage: 27% (15% hydro, 7% wind, 5% solar)
- global average fuel input ratio and output composition (per kWh)
| Fuel Type | Input (kg/kWh) | Composition ratio | conversion efficiency |
| coal | 0.25~0.30 | 36%. | 35-45% of |
| natural gas | 0.18~0.20 | 23%. | 50-60%. |
| petroleum | 0.02 to 0.03 | 3 | 25% to 35 |
| biomass | 0.45~0.55 | 3 | 20-30%. |
| nuclear power | 0.00003 | 10% (%) | 33-37%. |
| renewable energy | nashi (Pyrus pyrifolia, esp. var. culta) | 27% of | N/A |
- resource input and overview for 1 kWh generation
- Coal-fired power: 0.25-0.30 kg of coal is consumed and about 0.9 kg of CO₂ is emitted.
- Natural gas-fired: 0.18-0.20 kg of gas produces about 0.4 kg/kWh of CO₂ emissions.
- Nuclear power: Generates large amounts of energy from very small amounts of uranium, but requires waste management.
- Renewable energy: No fuel input, but requires resources for initial capital investment.
Points to keep in mind when manufacturing
- The fuel input ratio may change in the future due to the expansion of renewable energy sources.
- Fossil fuel reduction and waste management are important parts of addressing climate change.
Coal-fired power generation 1 kWh (considering equipment lifetime)
In coal-fired power generation, along with the fuel, the construction and operating resources of the facility are also involved in energy production. Below is a rough estimate of how those resources are allocated per kWh, taking into account the useful life of the facility (approximately 30 to 40 years).
- coal-fired power generation Substance input per kWh
1.1 Fueling
- Coal: 0.25-0.30 kg
- (calorific value 20-30 MJ/kg)
- By combustion: 0.9 kg CO₂ emissions
- Limestone (for desulfurization): 0.01-0.02 kg
- Used to reduce SO₂ emissions
- Water: 1.8 to 2.5 L
- For boiler steam generation and cooling
1.2 Allocation of Facility Construction and Maintenance Resources
- Steel and concrete:
- Steel: 0.01 to 0.02 kg/kWh
- Concrete: 0.05-0.1 kg/kWh
- (Used for construction of power plants, allocated over a 40-year life)
- Transport fuels (coal transportation):
- Diesel oil: 0.001-0.002 kg/kWh
- energy input and waste generation
energy input
- Electricity: 0.01-0.02 kWh/kWh
- (control systems, pumps, blower operation)
- Natural gas or fuel oil (for start-up and maintenance):
- Fuel: 0.005 to 0.01 kg/kWh
waste
- CO₂ emissions: 0.9-1.0 kg/kWh
- Coal ash: 0.04-0.05 kg/kWh
- Part of the material can be reused for concrete products, etc.
- Wastewater: 0.005 to 0.01 kg/kWh
- Effluent generated in the FGD process
Overview of manufacturing process
- Combustion: Coal is burned to produce steam.
- Exhaust gas treatment: Limestone is used to remove sulfur oxides.
- Cooling water circulation: Large amounts of water are used to cool the power plant.
- Waste Management: Some coal ash is reused, but most is landfilled.
- key points for considering the service life of coal-fired power generation
- Although the cost of maintaining the equipment is small, the amount of electricity generated means that the equipment resource input per kWh is negligible.
- Waste management (especially coal ash and flue gas) is an important process to reduce environmental impact.
- Increased energy efficiency: state-of-the-art supercritical power generation (supercritical steam technology) achieves more than 40% efficiency and reduces fuel use.
- summary of inputs per kWh for coal-fired power generation
| Input Substances | Amount (kg/kWh) | remarks |
| coal | 0.25~0.30 | main fuel |
| Limestone (desulfurization) | 0.01 to 0.02 | SO₂ emission control |
| water (esp. cool, fresh water, e.g. drinking water) | 1.8 to 2.5 L | Used for boiler and cooling |
| Steel & Concrete | 0.06 to 0.12 | Construction materials, allocated for 40 years of useful life |
| Diesel oil (transportation fuel) | 0.001 to 0.002 | For coal transportation |
| Natural gas or heavy oil | 0.005 to 0.01 | Equipment start-up and maintenance |
| Waste (coal ash) | 0.04 to 0.05 | Partially reused |
| CO₂ emissions | 0.9 to 1.0 | Combustion and process emissions |
Natural gas-fired power generation (considering equipment lifetime)
Natural gas-fired power generation is typically gas turbine or **Combined Cycle Generation (CCGT)**. High efficiency (50-60%) is achieved with combined cycle. Below are the inputs and their quantities, allocated per kWh of fuel and equipment materials, taking into account the lifetime of the facility (approximately 30-40 years).
- natural gas-fired power generation Substance input per kWh
1.1 Fueling
- Natural gas: 0.18-0.20 kg
- Heat value: 42-45 MJ/kg
- CO₂ emissions: 0.35-0.45 kg/kWh (depending on combustion)
- Water: 0.2 to 0.4 L
- For cooling and steam generation (for combined cycle power generation)
1.2 Allocation of Facility Construction and Maintenance Resources
- Steel and concrete:
- Steel: 0.005 to 0.01 kg/kWh
- Concrete: 0.02-0.05 kg/kWh
- (Allocated over 40 years of useful life)
- Diesel oil (for natural gas transportation and maintenance):
- 0.0005 to 0.001 kg/kWh
- energy input and waste generation
energy input
- Electricity: 0.01-0.02 kWh/kWh
- Used for system control, pump operation, compressors
- Natural gas or diesel oil (startup and maintenance):
- Fuel: 0.005 to 0.01 kg/kWh
waste
- CO₂ emissions: 0.35-0.45 kg/kWh
- Wastewater: 0.001-0.002 kg/kWh (effluent from cooling process)
Overview of manufacturing process
- Gas turbine or combined cycle power generation:
natural gas is burned to turn a turbine; in a combined cycle, waste heat is reused to produce steam. - Cooling and Exhaust Gas Treatment:
Cooling water is used as needed and catalysts are utilized to reduce NOx. - Maintenance of facilities:
Fuel transportation and light oil for maintenance are also used.
- natural gas-fired power generation Summary of inputs per kWh
| Input Substances | Amount (kg/kWh) | remarks |
| natural gas | 0.18~0.20 | main fuel |
| water (esp. cool, fresh water, e.g. drinking water) | 0.2 to 0.4 L | For cooling and steam generation |
| Steel & Concrete | 0.025~0.06 | Power plant construction materials (allocated over 40 years) |
| Diesel oil (transportation and maintenance) | 0.0005 to 0.001 | Used for maintenance and transportation |
| CO₂ emissions | 0.35~0.45 | Emissions from combustion |
| wastewater | 0.001 to 0.002 | Drainage of cooling process |
- points for consideration of service life
- Energy efficiency: Combined cycle technology allows for efficiencies as high as 50-60%, reducing fuel use.
- Exhaust gas control: Selective catalytic reduction (SCR) and other methods are used to reduce NOx emissions.
- Long-term use of equipment: Equipment materials are subdivided and allocated in anticipation of a 40-year service life.
- Energy reuse: Combined cycle power generation reuses waste heat to increase overall efficiency.
- environmental considerations
- CO₂ emissions reduction: Efficient combustion and exhaust heat recovery reduce CO₂ emissions.
- Efficient use of water: Cooling water is circulated whenever possible to reduce usage.
- Sustainability: Natural gas has lower emissions than coal, making it an effective short-term climate solution, but in the long term, a transition to renewable energy sources is desirable.
In this process, efficient use of fuel and reuse of waste heat are important to reduce costs and environmental impact. In addition, the installation of exhaust gas treatment equipment and cooling systems minimizes environmental impact.
Oil-fired power generation (taking into account the useful life of the equipment)
Oil-fired power generation is typically based primarily on diesel engines or gas turbines. The construction materials (steel, concrete, etc.) of the power plant are allocated to a useful life (approximately 30-40 years), and resource consumption is shown per kWh.
- oil-fired power generation Substance input per kWh
1.1 Fueling
- Heavy oil or light oil: 0.2-0.25 kg/kWh
- Heat value: 42-45 MJ/kg
- CO₂ emissions: 0.65-0.75 kg/kWh
- Water: 0.3 to 0.5 L
- Used for cooling and exhaust gas cleaning
1.2 Allocation of Facility Construction and Maintenance Resources
- Steel and concrete:
- Steel: 0.005 to 0.01 kg/kWh
- Concrete: 0.02-0.05 kg/kWh
- (Allocated over the 40-year life of the power plant)
- Diesel oil (for transportation and maintenance):
- 0.0005 to 0.001 kg/kWh
- energy input and waste generation
energy input
- Electricity: 0.01 to 0.03 kWh/kWh
- Used for system control, pumps and blowers
- Heavy or light oil (startup and maintenance):
- 0.005 to 0.01 kg/kWh
waste
- CO₂ emissions: 0.65-0.75 kg/kWh
- Exhaust gas: contains NOx and SOx
- Flue gas treatment facilities (desulfurization and denitrification)
- Wastewater: 0.01-0.02 kg/kWh (generated by exhaust gas cleaning)
Overview of manufacturing process
- Combustion and Power Generation:
Heavy or light oil is burned to generate electricity with a gas turbine or diesel engine. - Exhaust gas treatment:
Selective catalytic reduction (SCR) and desulfurization equipment are used to reduce NOx and SOx emissions. - Cooling and Maintenance:
Cooling water is used to cool the engine and turbine, and maintenance is performed on a regular basis.
- oil-fired power generation Summary of inputs per kWh
| Input Substances | Amount (kg/kWh) | remarks |
| Heavy oil or light oil | 0.2 to 0.25 | main fuel |
| water (esp. cool, fresh water, e.g. drinking water) | 0.3 to 0.5 L | For cooling and flue gas cleaning |
| Steel & Concrete | 0.025~0.06 | Construction materials (allocated over 40-year life) |
| Diesel oil (transportation and maintenance) | 0.0005 to 0.001 | For maintenance and fuel transportation |
| CO₂ emissions | 0.65~0.75 | Emissions from combustion |
| wastewater | 0.01 to 0.02 | Exhaust gas cleaning water |
- points for consideration of service life
- Fuel Use: Fuel efficiency is 30-40%.
- Flue gas treatment: Desulfurization and denitrification facilities are required to remove NOx and SOx.
- Energy optimization: Cooling water is circulated and waste heat is reused whenever possible.
- environmental considerations
- Emission control: NOx reduction by SCR (Selective Catalytic Reduction) and SOx reduction by desulfurization equipment are important.
- CO₂ emission reduction: Since oil has the highest emissions of all fossil fuels, its use should be avoided in the short term and a shift to renewable energy sources should be made.
- Efficient use of water resources: Reduces consumption of water resources by circulating cooling water.
In this process, the reduction of fuel consumption and proper operation of exhaust gas treatment are key to reducing environmental impact. Further cost reductions are also expected if the reuse and recycling of equipment materials during their useful life is promoted.
Biomass power generation (taking into account the useful life of equipment)
Biomass power generation generates electricity by burning and gasifying biomass resources such as wood pellets, agricultural waste, and food waste. Considering the useful life of the equipment (approximately 20 to 40 years), the following is a rough estimate of inputs, with fuels and materials allocated per kWh.
- biomass power generation Substance input per kWh
1.1 Fueling
- Wood pellets or biomass: 0.5-0.6 kg/kWh
- Calorific value: 16-20 MJ/kg
- CO₂ emissions: 0.2-0.3 kg/kWh (considered virtually zero due to carbon cycle)
- Water: 0.3 to 0.6 L
- Used for steam generation and cooling
1.2 Allocation of Facility Construction and Maintenance Resources
- Steel and concrete:
- Steel: 0.01 to 0.02 kg/kWh
- Concrete: 0.04-0.06 kg/kWh
- (Allocated over a useful life of 20 to 40 years)
- Diesel oil (for transportation and maintenance):
- 0.001~0.002 kg/kWh
- energy input and waste generation
energy input
- Electricity: 0.01-0.02 kWh/kWh
- Used for system control, pumps and blowers
- Diesel oil (fuel transportation and equipment start-up):
- 0.005 to 0.01 kg/kWh
waste
- Exhaust gas: Trace emissions of CO₂, NOx, and SOx
- Use catalytic reduction for NOx reduction
- Ash: 0.03-0.05 kg/kWh
- Some can be reused as agricultural fertilizer or construction material
Overview of manufacturing process
- Combustion or gasification:
Biomass fuel is burned to power a steam turbine. In the case of gasification, synthesis gas is burned to generate electricity. - Cooling and exhaust gas treatment:
Water is used to cool the steam, and exhaust gas is treated with a catalyst to reduce NOx. - Maintenance and upkeep:
Diesel fuel is used to transport fuel and maintain equipment.
- biomass power generation Summary of inputs per kWh
| Input Substances | Amount (kg/kWh) | remarks |
| Wood pellets or biomass | 0.5 to 0.6 | main fuel |
| water (esp. cool, fresh water, e.g. drinking water) | 0.3 to 0.6 L | For cooling and steam generation |
| Steel & Concrete | 0.05 to 0.08 | Facility construction materials (allocated over 20-40 years) |
| Diesel oil (transportation and maintenance) | 0.001 to 0.002 | For maintenance and transportation |
| CO₂ emissions | 0.2 to 0.3 | Considered virtually zero due to the carbon cycle |
| ash | 0.03 to 0.05 | Reuse for agricultural fertilizers and construction materials |
- points for consideration of service life
- Fuel sustainability: the use of sustainable forestry and agricultural waste is important.
- Reuse of ash: After combustion, ash is reused in agricultural fertilizers and cement products to reduce waste.
- Circulation of cooling water: Efficient use of water resources contributes to environmental protection.
- environmental considerations
- Exhaust gas control: Catalytic reduction method reduces NOx and improves combustion efficiency.
- CO₂ neutrality: Biomass emits CO₂ absorbed by plants during their growth, thus establishing a carbon cycle.
- Utilization of renewable energy sources: Biomass is a sustainable energy source as an alternative to fossil fuels.
In this process, efficient use of fuel and reuse of ash are key to sustainability and reduction of environmental impact. Long-term operation of the facility and the introduction of a circulating water system are expected to further improve efficiency.
Nuclear power generation (taking into account the service life of the equipment)
In nuclear power generation, uranium fuel is fissioned to turn a steam turbine to produce electricity. Since the equipment and construction materials for nuclear reactors are large and have a long service life (approximately 40 to 60 years), these resource inputs must also be allocated per kWh.
- nuclear power generation Substance input per kWh
1.1 Fueling
- Enriched uranium fuel (UO₂ pellets):
- Quantity: 0.00002 to 0.00003 kg/kWh
- Calorific value: Approximately 24,000 kWh can be generated from 1 g of uranium fuel
- Water: 0.5 to 1.0 L
- Used for steam generation and cooling
1.2 Allocation of Facility Construction and Maintenance Resources
- Steel and concrete:
- Steel: 0.01 to 0.015 kg/kWh
- Concrete: 0.05 to 0.10 kg/kWh
- (Allocated over a useful life of 40 to 60 years)
- Diesel oil (for fuel transportation and maintenance):
- 0.0005 to 0.001 kg/kWh
- energy input and waste generation
energy input
- Electricity: 0.005 to 0.01 kWh/kWh
- Used in control systems and cooling water circulation pumps
- Diesel oil or natural gas (for maintenance):
- 0.001~0.002 kg/kWh
waste
- Spent fuel: 0.000001 kg/kWh
- Requires long-term storage and reprocessing
- Wastewater: 0.001-0.002 L/kWh
- Part of the cooling water that needs to be controlled for radioactivity
Overview of manufacturing process
- Fission reaction:
Enriched uranium fuel is fissioned to produce a large amount of heat energy. - Steam generation and turbine drive:
The heat generated is used to produce steam, which turns a turbine to generate electricity. - Cooling system:
Steam is cooled and reused. Large volumes of water are used for cooling. -
Spent Fuel Management:
Spent fuel is stored in intermediate storage facilities and some is reprocessed.
- nuclear power generation Summary of inputs per kWh
| Input Substances | Amount (kg/kWh) | remarks |
| enriched uranium fuel | 0.00002 to 0.00003 | main fuel |
| water (esp. cool, fresh water, e.g. drinking water) | 0.5 to 1.0 L | For cooling and steam generation |
| Steel & Concrete | 0.06 to 0.12 | Construction materials (allocated over 40-60 years) |
| Diesel oil (for transportation and maintenance) | 0.0005 to 0.001 | Used for maintenance and transportation |
| spent fuel | 0.000001 | Storage and reprocessing required |
| wastewater | 0.001 to 0.002 L | Need to control radiation |
- points for consideration of service life
- Fuel efficiency: A small amount of uranium fuel can generate a large amount of electricity, resulting in a small resource input per kWh.
- Burden of equipment and materials: Nuclear power plants require large amounts of concrete and steel for construction, but the impact per kWh is small because they operate for long periods of time.
- Waste management: Spent fuel needs to be stored and reprocessed, and long-term management is an issue.
- environmental considerations
- Reduced CO₂ emissions: Since no CO₂ is emitted during power generation, the system is an effective alternative to fossil fuels.
- Management of wastewater and spent fuel: It is important to ensure thorough management of radioactivity.
- Sustainable Energy: Reprocessing of spent fuel and development of new fuel cycle technologies will increase sustainability.
While this process generates a large amount of power with a small amount of fuel, the treatment and long-term management of spent fuel is a challenge. In addition, the construction materials for the facility can be used for a long period of time with a single input, so the environmental impact per kWh is distributed.
Photovoltaic and wind power (considering equipment lifetime)
Photovoltaic and wind power generation do not use fuel during operation, so the materials required during construction and resources for maintenance are a burden per kWh. Below is a rough estimate of the input materials, taking into account the useful life of the facilities (30 years for solar and 20-25 years for wind) and allocating them per kWh.
- photovoltaic power generation Substance input per kWh
1.1 Allocation of Facility Construction and Maintenance Resources
- Silicon (for solar panels)
- Quantity: 0.01 to 0.015 kg/kWh
- Polycrystalline silicon and glass for panel production
- Aluminum Glass
- Aluminum: 0.003 to 0.005 kg/kWh
- Glass: 0.01 to 0.02 kg/kWh
- Concrete (for installation foundation)
- Quantity: 0.02 to 0.05 kg/kWh
- Diesel oil (for maintenance and transportation)
- Quantity: 0.0001 to 0.0002 kg/kWh
1.2 Energy input and waste
- Electricity: 0.005 to 0.01 kWh/kWh
- Used for inverters and management systems
- Waste:
- Waste panel: 0.0005-0.001 kg/kWh (at the end of service life)
- wind power generation Substance input per kWh
2.1 Allocation of Facility Construction and Maintenance Resources
- Steel and copper (for towers and generators)
- Steel: 0.01 to 0.02 kg/kWh
- Copper: 0.001-0.002 kg/kWh
- Concrete (foundation)
- Quantity: 0.05 to 0.1 kg/kWh
- Composite materials (for blades)
- Quantity: 0.002 to 0.005 kg/kWh
- Diesel oil (for maintenance and transportation)
- Quantity: 0.0005 to 0.001 kg/kWh
2.2 Energy input and waste
- Electricity: 0.005 to 0.01 kWh/kWh
- Used for control systems and maintenance equipment
- Waste:
- Waste blade: 0.0003 to 0.0005 kg/kWh (at the end of service life)
- renewable electricity Summary of input materials per kWh (average of solar and wind power)
| Input Substances | Amount (kg/kWh) | remarks |
| Silicon Aluminum | 0.01 to 0.02 | For solar panels |
| Steel & Copper | 0.01 to 0.02 | For wind towers and generators |
| concrete | 0.03 to 0.05 | For installation foundation |
| composite material | 0.002 to 0.005 | For wind power blades |
| Diesel oil (maintenance and transportation) | 0.0003 to 0.001 | Used for maintenance and transportation |
| electric power | 0.005 to 0.01 | Used for control systems and maintenance |
| waste | 0.0005 to 0.001 | End-of-life scrap (panels and blades) |
- points for consideration of service life
- Photovoltaic and wind turbines have a useful life of 30 and 20-25 years, respectively, and the environmental impact of construction materials is distributed.
- Optimize maintenance and transportation: remote management and preventive maintenance are important to reduce consumption of diesel fuel.
- Reuse of waste: Reuse and recycling of solar panels and wind blades is an issue.
- environmental considerations
- CO₂ emissions: CO₂ emissions during operation are almost zero. However, CO₂ emissions during material production and disposal are taken into account.
- Sustainability of materials: recycling technologies for renewable energy facilities need to be improved.
- Water use: Since no cooling water is used, the load on water resources is low.
Efficient use of construction materials and end-of-life waste management are key to reducing environmental impact in this process. Further sustainability is also achieved by increasing energy efficiency during operation.
coal
Coal is mined by open-pit or underground mining methods. Each mining method uses different inputs and different amounts of energy, but here we give an average of some general figures.
- power input and fuel to obtain 1 kg of coal
Input power
- Electricity:
- 0.03~0.05 kWh/kg
- (Used in mining equipment, conveyor belts, drainage pumps, etc.)
Input fuel
- Type: Diesel fuel (heavy equipment and transport vehicles)
- Amount: 0.02-0.05 kg/kg
- (Used for excavator and truck operations)
- CO₂ emissions other than combustion
- CO₂ emissions: 0.01-0.03 kg/kg
- (indirect emissions from mine operations, transportation, and wastewater treatment)
- inputs required for coal mining
- Name | Approximate amount of dosage
- Explosives (ANFO, TNT, etc.) | 0.001-0.002 kg
- Steel parts (equipment maintenance) | 0.0005-0.001 kg
- process solvents and auxiliaries
- Name | Usage | Circulation Rate
- Water (dust control and mine drainage management) | 2-5 L | 80-90
- Lubricating oil (for machine maintenance) | 0.0005 to 0.001 kg | Waste oil disposal after use
- waste requiring treatment
- Name | Approximate quantity
- Waste rock (rock debris/impurities) | 0.2-0.5 kg/kg
- Mine drainage | 0.5 to 1.0 L/kg
- Neutralization and treatment required if acid mine drainage (containing heavy metals) is included
- the amount of soil, water, and other resources that can be given change
- Soil and rock removal: 2-5 kg/kg
- (In the case of open pit mining, the upper bedrock and soil must be removed.)
- Water consumption: 5-10 L/kg
- (dust suppression, cooling, wastewater treatment, etc.)
- overview of coal mining process
- Drilling and Mining: Coal is mined by open-pit or underground mining. When explosives are used
The rock layers are then blasted to expose the coal beds.
- Coal recovery and transportation: Coal is dug out with heavy equipment and transported by truck or conveyor belt to a processing facility.
- Treatment of mine drainage: The discharged water is used to neutralize and purify acid mine drainage. If heavy metals are present, special treatment is required.
- Dust suppression: Water is sprayed to suppress dust during excavation and transportation.
- Waste Management: Waste rock generated during excavation will be considered for landfill or reuse.
- summary of inputs per kg of coal mined
| (data) item | Quantity (unit/kg coal) | remarks |
| electric power | 0.03 to 0.05 kWh | Mining equipment, drainage pumps, transport facilities, etc. |
| diesel fuel | 0.02 to 0.05 kg | Used for heavy equipment and transport trucks |
| Explosives (e.g. ANFO) | 0.001 to 0.002 kg | Used for blasting rock layers |
| Steel parts | 0.0005 to 0.001 kg | Used to maintain equipment |
| water (esp. cool, fresh water, e.g. drinking water) | 5-10 L | Used for dust suppression and wastewater treatment |
| lubricating oil | 0.0005 to 0.001 kg | Used for machine maintenance |
| Waste rock (impurities) | 0.2 to 0.5 kg | Occurred during mining |
| mine drainage | 0.5 to 1.0 L | May contain acid mine drainage |
| CO₂ emissions | 0.01 to 0.03 kg | Emissions from processes other than combustion |
| Amount of soil and stone removed | 2 to 5 kg | Open-pit mining |
- environmental considerations
- Treatment of acid mine drainage: Heavy metals and acidic water are properly neutralized to minimize environmental impact.
- Reuse of waste rock: Some waste rock is reused as construction material or for landfill.
- Water recycling: Water used for mining and cooling is reused as much as possible to reduce resource consumption.
Efficient use of fuel and proper management of wastewater and waste are key to environmental protection in this process. In addition, open-pit mining requires the reuse of land and environmental restoration after mining.
concrete
Estimates of inputs, energy consumption, and waste for the approximate production of 1 kg of concrete are given, considering current industrial processes.
Input power (kWh/kg)
0.01 – 0.03 kWh/kg
Electricity consumption during cement grinding and kneading processes.
- input fuel
Type: Coal, heavy oil, natural gas
Quantity:
Coal: 0.02 – 0.05 kg/kg
Heavy oil: 0.01 – 0.03 kg/kg
Natural gas: 0.02 – 0.04 kg/kg
Mainly used as fuel for cement firing process (rotary kiln).
3 CO₂ emissions other than combustion
CO₂ emissions from the decomposition of limestone (CaCO₃) in the manufacture of cement.
- the amount of change to the resource
Soil and aggregate: 1.5 – 1.8 kg/kg
(sand, crushed stone, aggregate, etc.)
Water: 0.15 – 0.2 kg/kg
used as mixing water.
- input materials and input volume guidelines
Cement: 0.25 – 0.30 kg
Sand: 0.4 – 0.5 kg
Crushed stone (aggregate): 0.7 – 0.8 kg
Water: 0.15 – 0.2 kg
Admixture (e.g. plasticizer): 0.001 – 0.005 kg
- process substances such as solvents
Name: Admixture (water reducer, plasticizer), setting retarder
Usage: 0.001 – 0.005 kg/kg
Circulation rate: low (much is consumed in the process)
- wastes and their approximate quantities
Name: Residual water (washing water), waste cement
Approximate quantity: 0.01 – 0.03 kg/kg
(waste water from cleaning process and disposal of unused cement)
fuel oil
Fuel oil is primarily a byproduct of the crude oil distillation process and is considered an energy and resource input throughout the refining process.
Input power (kWh/kg)
0.01 – 0.05 kWh/kg
(used for pump operation, distillation unit control, and process monitoring systems)
- input fuel
Type: Natural gas, electricity, petroleum coke
Approximate quantity:
Natural gas: 0.005 – 0.01 kg/kg
Petroleum coke: 0.002 – 0.005 kg/kg
They are mainly used in distillation columns and heating furnaces.
3 CO₂ emissions other than combustion (kg)
0.01 – 0.03 kg/kg
(secondary emissions from chemical reactions, e.g
. hydrogenation process)
(e.g. hydrogenation process)
- amount of change to the resource (kg)
Water: 0.3 – 0.5 kg/kg
(used as cooling water or process water)
Crude oil: 1.05 – 1.2 kg/kg
(heavy oil is a fractional distillate product of crude oil)
- input materials and input volume guidelines
Crude oil: 1.05 – 1.2 kg
Catalyst (e.g. hydrogenation catalyst): 0.001 – 0.002 kg
Hydrogen: 0.005 – 0.01 kg (depending on case)
- process substances such as solvents
Name: Additives (antioxidants, stabilizers, etc.), hydrogen
Usage: 0.005 – 0.01 kg/kg
Circulation rate: 20 – 50% (hydrogen is partially recovered and reused)
- waste requiring special treatment
Name: Refining sludge, waste catalyst
Approximate quantity: 0.01 – 0.02 kg/kg
(sludge must be treated, catalyst may be renewable)
Reference Process and Background
Heavy fuel oil is obtained from the atmospheric distillation and vacuum distillation processes of crude oil. The resulting heavy oil is used as marine and industrial fuel.
The plant uses large volumes of water in the cooling system and hydrogenation is part of the process.
Uranium fuel (enriched uranium)
Uranium enrichment is very energy-intensive and is mainly carried out by gaseous diffusion or centrifugation, but is based here on the more common centrifugation method.
Input power (kWh/kg)
2000 – 2500 kWh/kg
(consumed during the concentration process by centrifugation. Power consumption depends on the efficiency of the facility.)
- input fuel
Type: Mainly electricity (natural gas and oil may be used in some cases as backup fuel)
Quantity:
Natural gas: 0.01 – 0.02 kg/kg
(if used to supply electricity)
3 CO₂ emissions other than combustion
0.05 – 0.1 kg CO₂/kg
(generated in chemical reactions such as in the uranium fluoride (UF₆) production process)
- the amount of change to the resource
Water: 1.5 – 2.0 kg/kg
(used as cooling and process water)
Uranium ore: approx. 10 – 15 kg/kg
(uranium enrichment requires refining and conversion from ore)
- input materials and input volume guidelines
Natural uranium (UF₆): approx. 7 – 8 kg
(input for producing low-enriched uranium (LEU) with an enrichment of 3-5%)
Fluorine: 0.5 – 1.0 kg
(used to produce UF₆)
- process substances such as solvents
Name: Coolants and lubricants
Coolant (water): 1.5 – 2.0 kg/kg
Circulation rate: Cooling water circulation rate is 80-90%.
- waste requiring special treatment
Name: depleted uranium (DU), spent fluorine compounds
Approximate quantity: 6 – 7 kg/kg
(depleted uranium needs to be reused or stored for a long time)
Spent catalysts and solvents: 0.01 – 0.02 kg/kg
Reference Process and Background
Uranium fuel fabrication involves three processes: (1) refining from uranium ore, (2) conversion to UF₆, and (3) enrichment by centrifugal separation.
Depleted uranium (DU) is a byproduct of the enrichment process and may be stored as nuclear fuel or material for military use.
Silicon for photovoltaic (PV) power generation
Silicon for PV is typically produced as high-purity polysilicon (polycrystalline or monocrystalline silicon). The process is energy-intensive, and primary methods include the Siemens method.
Input power (kWh/kg)
70 – 100 kWh/kg
(reduction and refining process by Siemens method)
- input fuel
Type: Electricity, natural gas (for heating), hydrogen
Approximate quantity:
Natural gas: 0.02 – 0.05 kg/kg
Hydrogen: 0.001 – 0.003 kg/kg
(used for reduction processes and furnace heating)
3 CO₂ emissions other than combustion
0.5 – 1.2 kg CO₂/kg
(secondary emissions from chemical reactions and processes)
- the amount of change to the resource
Water: 5 – 10 kg/kg
(used for cooling and cleaning)
Quartz sand (SiO₂): 2.5 – 3.0 kg/kg
(used for reduction to metallic silicon)
- input materials and input volume guidelines
High purity quartz sand: 2.5 – 3.0 kg
Hydrogen chloride (HCl): 0.5 – 1.0 kg
(required for silane gas synthesis)
Hydrogen gas (H₂): 0.001 – 0.003 kg
Trichlorosilane (SiHCl₃): Process intermediate
- process substances such as solvents
Name: Hydrochloric acid, solvent for cleaning
Usage: 0.2 – 0.5 kg/kg
Recirculation rate: 50 – 80% (some hydrochloric acid and solvents are recirculated)
- waste requiring special treatment
Name: Silicon powder (residue from crushing and processing), acid treatment waste
Approximate quantity: 0.1 – 0.2 kg/kg
(some powder can be reused, acid waste requires appropriate treatment)
Reference Process and Background
Siemens method: High-purity silicon is obtained by reducing SiHCl₃ (trichlorosilane). Uses a very high-temperature furnace and requires large amounts of electricity and cooling water.
Generation and recirculation of trichlorosilane: Silicon compounds produced in the manufacturing process are partially recirculated and used as raw materials.
aluminum plate
Aluminum is generally produced from bauxite to produce alumina (Al₂O₃), followed by an electrolytic process to obtain metallic aluminum, which is then rolled. The following data is based on an aluminum production process using the common Hall-Héroult method (Hall-Héroult method).
Input power (kWh/kg)
13 – 15 kWh/kg
(electricity consumption in electrolytic reduction of alumina)
- input fuel
Type: Natural gas, coal, heavy oil
Approximate quantity:
Natural gas: 0.02 – 0.05 kg/kg
Heavy oil or coal: 0.01 – 0.03 kg/kg
(used in power generation and melting and rolling processes)
3 CO₂ emissions other than combustion
0.5 – 1.0 kg CO₂/kg
(generated by wear and tear of carbon electrodes and use of fluorine compounds)
- the amount of change to the resource
Bauxite ore: 4 – 5 kg/kg
(raw material to produce 1 kg of aluminum)
Water: 1 – 2 kg/kg
(used for refining process and cooling)
- input materials and input volume guidelines
Alumina (Al₂O₃): 1.9 – 2.0 kg
Carbon electrode: 0.4 – 0.5 kg
(consumed in the electrolysis process)
- process substances such as solvents
Name: Aluminum bromide (AlF₃), Calcium bromide (CaF₂)
Usage: 0.02 – 0.05 kg/kg
(used for molten salt in the electrolyzer)
Circulation rate: 50 – 80% (some solvents are recycled)
- waste requiring special treatment
Name: Red mud (bauxite residue), spent carbon electrodes
Approximate quantity: 1.5 – 2.0 kg/kg (red mud)
(requires proper treatment and landfill)
Carbon electrode waste: 0.1 – 0.2 kg/kg
(consumable portion of electrode)
Reference Process and Background
Hall-Elou method: Aluminum is produced from alumina using a molten salt electrolysis process. This method is very power intensive and poses a major challenge to the sustainability of the aluminum manufacturing industry.
Red mud problem: Red mud produced in the alumina manufacturing process requires landfill disposal, and technology is needed to reduce its impact on the environment.
Glass (common soda lime glass)
This data assumes that flat glass is mainly manufactured using the **float method**.
Input power (kWh/kg)
0.4 – 0.6 kWh/kg
(consumed by the control unit and cooling system during the mixing and melting process)
- input fuel
Type: Natural gas, heavy oil
Approximate quantity:
Natural gas: 0.18 – 0.25 kg/kg
Heavy oil: 0.02 – 0.05 kg/kg
(used for melting glass in kilns)
3 CO₂ emissions other than combustion
0.1 – 0.2 kg CO₂/kg
(generated by decomposition reaction of sodium carbonate, etc.)
- the amount of change to the resource
Sand (quartz sand): 1.2 – 1.4 kg/kg
Limestone: 0.1 – 0.15 kg/kg
Soda ash (sodium carbonate): 0.15 – 0.2 kg/kg
Water: 0.2 – 0.5 kg/kg (used for cooling and cleaning)
- input materials and input volume guidelines
Quartz sand (SiO₂): 1.2 – 1.4 kg
Sodium carbonate (Na₂CO₃): 0.15 – 0.2 kg
Limestone (CaCO₃): 0.1 – 0.15 kg
Dolomite (CaMg(CO₃)₂): 0.05 – 0.1 kg
- process substances such as solvents
Name: Cooling water, cleaning water
Usage: 0.2 – 0.5 kg/kg
Recirculation rate: 80 – 90% (most of the water is recirculated)
- waste requiring special treatment
Name: cullet (glass waste from manufacturing process), wastewater
Approximate quantity: 0.05 – 0.1 kg/kg
(cullet is reused, effluent needs treatment)
Reference Process and Background
In the float process, glass is melted at high temperatures and formed flat by spreading over a suspended tin surface. This process consumes large amounts of both fuel and electricity.
Cullet utilization: Glass waste (cullet) generated in the manufacturing process is re-input, thus improving resource use efficiency.
Wastewater treatment: Some of the cooling water and cleaning water is reused, but the discharge water needs to be treated.
copper
Copper production involves smelting from ores (primarily copper sulfide ores) and electrolytic refining. This section is based on typical pyrometallurgical and electrolytic smelting processes.
Input power (kWh/kg)
1.8 – 3.5 kWh/kg
(consumed mainly in the electrolytic refining process)
)
- input fuel
Type: Heavy oil, natural gas, coal
Approximate quantity:
Heavy oil: 0.01 – 0.02 kg/kg
Natural gas: 0.01 – 0.03 kg/kg
Coal or coke: 0.02 – 0.05 kg/kg
(used in melting furnace heating and reduction processes)
3 CO₂ emissions other than combustion
0.5 – 1.0 kg CO₂/kg
(generated by chemical reactions in the process, especially oxidation of sulfide ores)
- the amount of change to the resource
Copper ore: 100 – 200 kg/kg
(depending on the grade of copper in the ore)
Water: 10 – 15 kg/kg
(for refining and cooling)
- input materials and input volume guidelines
Copper ore (sulfide ore, oxide ore): 100 – 200 kg
Flux (e.g. silica sand): 0.5 – 1.0 kg
Oxygen: 0.1 – 0.2 kg
(Oxygen spraying improves furnace efficiency)
- process substances such as solvents
Name: Sulfuric acid, cleaning water
Amount used: Sulfuric acid: 0.2 – 0.5 kg/kg (used in electrolytic refining)
Circulation rate: Sulfuric acid: 80 – 90% (reused)
- waste requiring special treatment
Name: Slag, wastewater (containing sulfuric acid), dust
Approximate quantity:
Slag: 2 – 3 kg/kg
Wastewater: 0.1 – 0.2 kg/kg
(slag may be reused for building materials)
Reference Process and Background
In pyrometallurgical smelting, sulfide ores are melted and separated into matte and slag. The copper matte is then refined in a converter to obtain crude copper.
In electrolytic refining, crude copper is refined in an electrolytic bath to obtain copper with a purity of 99.99% or higher. Electrolytic solutions using sulfuric acid are widely used.
Composite materials for wind power blades
Below are the approximate inputs, energy, and waste for producing 1 kg of composite materials (primarily glass fiber reinforced plastic (GFRP) and carbon fiber reinforced plastic (CFRP)) used in wind power blades. Because blades are made of lightweight, high-strength materials, more resins, fibers, and energy for the process are used.
Input power (kWh/kg)
10 – 15 kWh/kg
(resin curing, fiber processing, lamination process, and operation of molding equipment)
- input fuel
Type: Natural gas, heavy oil
Approximate quantity:
Natural gas: 0.01 – 0.03 kg/kg
Heavy oil: 0.005 – 0.01 kg/kg
(used as heat source for molding process and drying furnace)
3 CO₂ emissions other than combustion
1.5 – 3.0 kg CO₂/kg
(generated in the resin and fiber production process)
- the amount of change to the resource
Ore (silicate for fiberglass): 1.2 – 1.5 kg/kg
Water: 2 – 3 kg/kg (for cooling and cleaning)
Fossil resource (for carbon fiber): 0.4 – 0.6 kg/kg
- input materials and input volume guidelines
Glass fiber: 0.5 – 0.7 kg
Epoxy resin: 0.3 – 0.5 kg
Hardener: 0.02 – 0.05 kg
Carbon fiber (depending on case): 0.1 – 0.2 kg
- process substances such as solvents
Name: Acetone, ethanol (for cleaning)
Usage: 0.01 – 0.02 kg/kg
Circulation rate: 50 – 70% (partially collected and reused)
- waste requiring special treatment
Name: Cutting scrap, excess resin, spent solvent
Approximate quantity: 0.1 – 0.2 kg/kg
(Cuttings are difficult to recycle and are mainly disposed of in landfills)
Reference Process and Background
Molding Methods: Vacuum molding and **RTM (resin injection molding)** are often used in wind blade manufacturing. These processes are complex and require skilled techniques.
Waste challenge: Disposal of excess glass and carbon fiber is a problem, and sustainable recycling methods are being sought.
sand
Sand mainly for construction and glass production is extracted from river, mountain, and sea sand, which is sieved and washed after mining.
Input power (kWh/kg)
0.005 – 0.01 kWh/kg
(used to run mining equipment, conveyors, sieving equipment, washing machines)
- input fuel
Type: Light oil, natural gas
Approximate quantity:
Diesel oil: 0.005 – 0.01 kg/kg (fuel for excavators and dump trucks)
Natural gas: 0.001 – 0.003 kg/kg (used for drying and some heating processes)
3 CO₂ emissions other than combustion
0.001 – 0.003 kg CO₂/kg
(indirect emissions from washing water and transportation)
- the amount of change to the resource
Soil and rock (rock and soil removed during excavation): 1.1 – 1.3 kg/kg
Water: 0.5 – 1.0 kg/kg (used for washing and grain size adjustment)
- input materials and input volume guidelines
Sand (to be mined): 1.1 – 1.3 kg (after mining, sieving to adjust yield)
Filter material: 0.01 – 0.02 kg (used in cleaning system)
- process substances such as solvents
Name: Cleaning water
Usage: 0.5 – 1.0 kg/kg
Circulation rate: 50 – 70% (part of the cleaning water is reused)
- waste requiring special treatment
Name: Sludge, excavated soil
Approximate quantity: 0.1 – 0.3 kg/kg
(mud must be landfilled or treated)
Reference Process and Background
Mining methods: suction pumps from rivers and the sea, and excavators are used for mining mountain sand. The quality of the sand extracted is adjusted by sieving and washing.
Waste management: Excavated soil residues and sludge after washing are properly disposed of according to environmental regulations.
Crushed stone (for construction materials)
After mining, the crushed stone is crushed and sieved to prepare it for transport.
Input power (kWh/kg)
0.005 – 0.01 kWh/kg
(used to run crusher and sieving machine)
- input fuel
Type: Light oil, heavy oil
Approximate quantity:
Diesel oil: 0.01 – 0.02 kg/kg
(used in excavators, dump trucks, etc. for excavation and transportation)
Heavy oil: 0.001 – 0.005 kg/kg
(used in some generators and heating processes)
3 CO₂ emissions other than combustion
0.002 – 0.005 kg CO₂/kg
(indirect emissions related to dust suppression and transportation)
- the amount of change to the resource
Soil and rocks (surrounding soil and unwanted rocks): 1.2 – 1.5 kg/kg
(generated during mining and separation of crushed stone)
Water: 0.1 – 0.3 kg/kg
(used for dust suppression and cleaning)
- input materials and input volume guidelines
Stone: 1.2 – 1.5 kg
(after crushing, yield is adjusted to obtain 1 kg product)
- process substances such as solvents
Name: Cleaning water, dust suppressant
Amount used: Water: 0.1 – 0.3 kg/kg
Circulation rate: 50 – 70% (wash water is often reused)
- waste requiring special treatment
Name: Dust, excavation overburden
Approximate quantity: 0.05 – 0.2 kg/kg
(dust needs to be controlled, residual soil can be used for landfill or land preparation)
Reference Process and Background
Mining Method: Open-pit mining is common, with excavators and crushers used for large-scale crushing.
Transportation: Transportation from the mining site to the processing plant or construction site consumes a lot of energy.
Environmental Impact: Control of dust and wastewater is important and must comply with regulations.
Admixture (e.g., water reducers and plasticizers for concrete)
Admixtures are produced through chemical processes, and depending on the type, energy consumption and management of by-products are important.
Input power (kWh/kg)
0.5 – 2.0 kWh/kg
(used for agitation of reaction vessel, temperature control, and operation of drying equipment)
- input fuel
Type: Natural gas, electricity (indirect use), heavy oil
Approximate quantity:
Natural gas: 0.01 – 0.03 kg/kg (for heating process)
Heavy oil: 0.005 – 0.01 kg/kg (if used in some drying facilities)
3 CO₂ emissions other than combustion
0.1 – 0.3 kg CO₂/kg
(emissions from chemical processes and solvent volatilization during reaction)
- the amount of change to the resource
Water: 0.5 – 1.0 kg/kg
(used for reaction systems and cleaning processes)
Chemical raw materials: 0.2 – 0.5 kg/kg
(including catalysts and additives)
- input materials and input volume guidelines
Main raw materials (e.g. naphthalene compounds, polycarboxylic acid ethers): 0.7 – 0.9 kg
Catalyst (e.g., acid catalyst, basic catalyst): 0.01 – 0.05 kg
Stabilizer/preservative: 0.01 – 0.03 kg
- process substances such as solvents
Name: Organic solvents (water, methanol, ethanol)
Usage: 0.1 – 0.3 kg/kg
Circulation rate: 50 – 80% (part of solvent is distilled and reused)
- waste requiring special treatment
Name: Reaction byproducts, spent solvents, waste liquids
Approximate quantity: 0.05 – 0.1 kg/kg
(effluent must be disposed of properly)
Reference Process and Background
Manufacturing Process: Many admixtures are produced by chemical reactions, and temperature control is critical. Stabilizers and preservatives are added to maintain quality after production.
Environmental Impact: Byproducts and spent solvents generated by the reaction must be reused or disposed of properly to avoid environmental impact.
Hydrogenation catalyst (e.g., palladium catalyst, ruthenium catalyst, etc.)
These catalysts are produced by a process in which precious metals are dispersed and immobilized on a support (activated carbon, alumina, etc.).
Input power (kWh/kg)
5 – 15 kWh/kg
(electricity for stirring, drying, firing and filtration processes)
- input fuel
Type: Natural gas, electricity (indirect use), heavy oil
Approximate quantity:
Natural gas: 0.02 – 0.05 kg/kg (used in heating processes for firing and drying)
Fuel oil: 0.005 – 0.01 kg/kg (auxiliary fuel for furnaces and drying equipment)
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.1 – 0.3 kg CO₂/kg
(emissions from chemical reaction by-products and solvent and catalyst production)
- the amount of change to resources such as earth, rocks, and water
Water: 1 – 3 kg/kg (used for cleaning and cooling)
Precious metal ores: 0.05 – 0.1 kg/kg (palladium, platinum, ruthenium, etc.)
- input materials and input volume guidelines
Precious metal compounds (e.g. PdCl₂, RuCl₃): 0.05 – 0.1 kg
Carrier (activated carbon, γ-alumina, etc.): 0.9 – 0.95 kg
Reducing agent (e.g. hydrogen gas): 0.01 – 0.05 kg
- process substances such as solvents
Name: Organic solvent (water, methanol, ethanol)
Usage: 0.1 – 0.3 kg/kg
Circulation rate: 50 – 80% (some of the materials are collected and reused)
- waste requiring special treatment
Name: Spent solvent, reaction byproducts, filtration residue
Approximate quantity: 0.05 – 0.1 kg/kg
(solvents and byproducts require proper disposal)
Reference Process and Background
Manufacturing process: Precious metal compounds are dissolved and dispersed on a support, then hydrogen and reducing agents are used to activate the catalyst. It is then dried and calcined to complete the process.
Environmental Considerations: Recycling technology is important due to the high use of precious metals and organic solvents. In addition, filtration residues are subject to waste management.
Natural uranium concentrate (yellowcake: U₃O₈)
The production of uranium concentrates involves the mining of uranium ore, crushing and leaching processes, and refining and drying processes.
Input power (kWh/kg)
0.1 – 0.5 kWh/kg
(used to run crushers, pumps, drying equipment, etc.)
- input fuel
Type: Light oil, electricity, natural gas
Approximate quantity:
Diesel oil: 0.02 – 0.05 kg/kg (used in mining and transportation)
Natural gas: 0.01 – 0.03 kg/kg (used as heat source for drying furnaces)
CO₂ emissions other than combustion (kg CO₂/kg)
0.01 – 0.05 kg CO₂/kg
(indirectly emitted by chemical processing from uranium ore)
- amount of change in resources such as soil, stone, and water (kg)
Ore: 100 – 200 kg/kg (uranium content 0.1 – 0.5%)
Water: 1.5 – 3 kg/kg (used as leachate)
- input materials and input volume guidelines
Sulfuric acid: 1 – 2 kg
(for leaching uranium)
Oxidants (e.g., hydrogen peroxide, nitric acid): 0.05 – 0.1 kg
(to accelerate leaching)
- process substances such as solvents
Name: Organic solvent (e.g., kerosene, triethylamine)
Usage: 0.1 – 0.2 kg/kg
Circulation rate: 50 – 70% (some solvents are recovered and reused)
- waste requiring special treatment
Name: Tailings (ore residue), acidic liquid waste
Approximate quantity: 1 – 5 kg/kg (tailings)
Acidic liquid waste: 0.5 – 1 kg/kg (requires appropriate treatment)
Reference Process and Background
Leaching method: Uranium is eluted from uranium ore using sulfuric acid and purified by precipitation or solvent extraction.
Tailings management: After mining, ore residue is generated in large quantities and must be properly managed because it contains radioactivity.
Environmental Impact: Acidic effluents and tailings are managed in accordance with environmental regulations.
Fluorine (F₂)
The industrial production of fluorine is primarily done through the electrolysis process of hydrogen fluoride (HF). This is a very energy-intensive process and requires proper controls because of the powerful chemicals involved.
Input power (kWh/kg)
40 – 60 kWh/kg
(electricity for generating fluorine by electrolysis of HF)
- input fuel
Type: Electricity, natural gas
Approximate quantity:
Natural gas: 0.01 – 0.03 kg/kg
(used for cooling and some heat sources)
CO₂ emissions other than combustion (kg CO₂/kg)
0.01 – 0.05 kg CO₂/kg
(indirect emissions from HF production and fluorine side reactions)
- amount of change in resources such as soil, stone, and water (kg)
Fluorite (CaF₂): 2 – 3 kg/kg
(used as raw material for HF)
Water: 1 – 2 kg/kg
(for process cooling)
- input materials and input volume guidelines
Hydrogen fluoride (HF): 2 – 2.5 kg
(decomposes into fluorine and hydrogen by electrolysis)
Potassium fluoride (KF): 0.5 – 1.0 kg
(used as stabilizer for electrolyte)
- process substances such as solvents
Name: Water, electrolyte (KF solution)
Usage: 1 – 2 kg/kg
Recirculation rate: 50 – 80% (part of the electrolyte can be recirculated)
- waste requiring special treatment
Name: Spent electrolyte, reaction residue
Approximate quantity: 0.2 – 0.5 kg/kg
(requires proper reprocessing of electrolyte)
Reference Process and Background
Production process: HF is electrolyzed to produce fluorine and hydrogen. Fluorine is extremely reactive and requires special equipment for production and storage.
Environmental Impact: Proper treatment of spent electrolyte and reaction residues is required, and effluents are managed according to hazardous substance regulations.
DU (Depleted Uranium) Isolation of rock salt mine sites
Sequestration to a rock salt mine site is intended to provide long-term stable storage of uranium, and the delivery and containment process consumes electricity and fuel. The following data are estimates based on typical industrial processes associated with transport, installation, and containment.
Input power (kWh/kg)
0.3 – 0.8 kWh/kg
(used for lighting, ventilation, operation of containment equipment, transfer cranes, etc.)
- input fuel
Type: Light oil, natural gas
Approximate quantity:
Diesel oil: 0.02 – 0.05 kg/kg
(used in transport vehicles and for underground transportation)
Natural gas: 0.005 – 0.01 kg/kg
(partially used for heating and ventilation systems)
CO₂ emissions other than combustion (kg CO₂/kg)
0.005 – 0.02 kg CO₂/kg
(indirect emissions from concrete encapsulation and sub-material production)
- amount of change in resources such as soil, stone, and water (kg)
Concrete or bentonite: 0.5 – 1.0 kg/kg
(used as containment)
Water: 0.1 – 0.3 kg/kg
(used for cooling and dust suppression)
- input item name and input amount guideline
Depleted uranium (DU): 1.0 kg
Encapsulant (concrete, bentonite): 0.5 – 1.0 kg
Reinforcement (e.g. steel liner): 0.05 – 0.1 kg
- process substances such as solvents
Name: Water, bentonite suspension
Usage: 0.1 – 0.2 kg/kg
Circulation rate: 30 – 50% (some water is reused)
- waste requiring special treatment
Name: Packaging materials, dust from construction
Approximate quantity: 0.02 – 0.05 kg/kg
(packaging materials are collected and processed, dust is controlled)
Reference Process and Background
Sequestration to rock salt mine sites: Rock salt formations are used to sequester radioactive materials due to their high long-term geologic stability.
Sealing process: Sealing with concrete or bentonite to prevent contact with surrounding water and air.
Transport and storage controls: Radiation shielding and monitoring during transport is required.
Long-term sequestration of depleted uranium (DU) is evaluated by considering the half-life of U-238, the major isotope of uranium.
- half-life and the need for prolonged isolation
Half-life of U-238: 446.8 billion years
Time to decay to 1/10,000th:
– Radioactive materials decrease their radioactivity through a series of half-lives. - To decay to 1/10,000th requires about 16 half-lives.
446.8 billion years x 16 ≈ 7.15 billion years 446.8 billion years x 16 ≈ 7.15 billion years
This should safely sequester depleted uranium for 7.15 billion years.
Total evaluation of the isolation process
The increase in electricity consumption, inputs, CO₂ emissions, and waste due to long-term sequestration must be evaluated cumulatively. Here, we model primarily assuming that management and maintenance costs are cumulative over the entire sequestration period.
Long-term average annual maintenance assumptions
Power and fuel consumption for annual inspection and maintenance
Materials consumed and waste generated on average per year
Fuel use for transportation and additional management and additional CO₂ emissions
7.15 billion year cumulative managed valuation (assumed value with annual maintenance)
Input power (kWh/kg)
Annual maintained power: 0.05 kWh/kg
For operating lighting, ventilation, and monitoring equipment
0.05 kWh/kg x 7.15 x 109 years = 3.575 x 108 kWh/kg 0.05 kWh/kg x 7.15 x 109 years = 3.575 x 108 kWh/kg
- input fuel
Annual fuel consumption (light oil): 0.001 kg/kg
(used for periodic inspections and transportation)
0.001 kg/kg x 7.15 x 109 years = 7.15 x 106 kg/kg 0.001 kg/kg x 7.15 x 109 years = 7.15 x 106 kg/kg
3 CO₂ emissions other than combustion
Annual CO₂ emissions (inspection and maintenance): 0.0001 kg CO₂/kg
Secondary emissions from monitoring facilities and routine operations
0.0001 kg/kg x 7.15 x 109 years = 7.15 x 105 kg CO₂/kg 0.0001 kg/kg x 7.15 x 109 years = 7.15 x 105 kg CO₂/kg
- the amount of change to the resource
Annual consumable materials (concrete, bentonite): 0.001 kg/kg
(replenishment of sealant and inspection and repair)
0.001 kg/kg x 7.15 x 109 years = 7.15 x 106 kg/kg 0.001 kg/kg x 7.15 x 109 years = 7.15 x 106 kg/kg
- waste requiring special treatment
Annual waste (packaging, consumables): 0.0001 kg/kg
0.0001 kg/kg x 7.15 x 109 years = 7.15 x 105 kg/kg 0.0001 kg/kg x 7.15 x 109 years = 7.15 x 105 kg/kg
Total Result (7.15 billion annual cumulative value)
| (data) item | Value (accumulated/kg) |
| electricity consumption | 3.575 × 10⁸ kWh/kg |
| Diesel oil consumption | 7.15 × 10⁶ kg/kg |
| CO₂ emissions other than combustion | 7.15 × 10⁵ kg CO₂/kg |
| Wear and tear of materials (e.g. concrete) | 7.15 × 10⁶ kg/kg |
| Waste (e.g., packaging materials) | 7.15 × 10⁵ kg/kg |
Conclusion.
The 7.15 billion year sequestration process requires energy and material inputs over an extremely long period of time. On such a time scale, sustainability of power and fuel consumption is a major challenge. In addition, costs are expected to be enormous, as annual inspections, material replenishment, and waste management are essential.
Below are the cumulative energy and material requirements for sequestering 1 kg of depleted uranium in a rock salt mine site over a 500-year period.
Average annual consumption (assumed)
Electricity: 0.05 kWh/kg
Fuel (light oil): 0.001 kg/kg
CO₂ emissions other than combustion: 0.0001 kg CO₂/kg
Material consumption (concrete and bentonite): 0.001 kg/kg
Waste (packaging materials and consumables): 0.0001 kg/kg
Cumulative calculations over 500 years
- power consumption
0.05 kWh/kg/year×500 years=25 kWh/kg0.05kWh/kg/year×500years=25kWh/kg
- diesel oil consumption
0.001 kg/kg/year×500 years=0.5 kg/kg0.001 kg/kg/year×500years=0.5 kg/kg
CO₂ emissions other than combustion
0.0001 kg CO₂/kg/year×500 years=0.05 kg CO₂/kg0.0001 kg CO₂/kg/year×500 years
- wear and tear of materials (concrete and bentonite)
0.001 kg/kg/year×500 years=0.5 kg/kg0.001 kg/kg/year×500years=0.5 kg/kg
- waste generation
0.0001 kg/kg/year×500 years=0.05 kg/kg0.0001 kg/kg/year×500years=0.05 kg/kg
Cumulative results over 500 years
| (data) item | Value (accumulated/kg) |
| electricity consumption | 25 kWh/kg |
| Diesel oil consumption | 0.5 kg/kg |
| CO₂ emissions other than combustion | 0.05 kg CO₂/kg |
| Material Consumption | 0.5 kg/kg |
| waste | 0.05 kg/kg |
Conclusion.
Even after 500 years of management, the consumption of electricity, fuel, and materials is cumulative, but not extremely large. In particular, the cumulative energy required to sequester 1 kg of depleted uranium is about 25 kWh/kg and 0.5 kg/kg of consumable materials.
Below are the cumulative energy and material requirements for sequestering 1 kg of depleted uranium in a rock salt mine site over a 1000-year period.
Average annual consumption (assumed)
Electricity: 0.05 kWh/kg
Fuel (light oil): 0.001 kg/kg
CO₂ emissions other than combustion: 0.0001 kg CO₂/kg
Material consumption (concrete and bentonite): 0.001 kg/kg
Waste (packaging materials and consumables): 0.0001 kg/kg
Cumulative calculations over 1000 years
- power consumption
0.05 kWh/kg/year×1000 years=50 kWh/kg0.05kWh/kg/year×1000years=50kWh/kg
- diesel oil consumption
0.001 kg/kg/year×1000 years=1.0 kg/kg0.001 kg/kg/year×1000years=1.0 kg/kg
CO₂ emissions other than combustion
0.0001 kg CO₂/kg/year×1000 years=0.1 kg CO₂/kg0.0001 kg CO₂/kg/year×1000years
- wear and tear of materials (concrete and bentonite)
0.001 kg/kg/year×1000 years=1.0 kg/kg0.001 kg/kg/year×1000years=1.0 kg/kg
- waste generation
0.0001 kg/kg/year×1000 years=0.1 kg/kg0.0001 kg/kg/year×1000years=0.1 kg/kg
Cumulative results over 1000 years
| (data) item | Value (accumulated/kg) |
| electricity consumption | 50 kWh/kg |
| Diesel oil consumption | 1.0 kg/kg |
| CO₂ emissions other than combustion | 0.1 kg CO₂/kg |
| Material Consumption | 1.0 kg/kg |
| waste | 0.1 kg/kg |
Conclusion.
For a 1000-year control, the cumulative energy and materials required to isolate 1 kg of depleted uranium would be as follows
Power consumption: 50 kWh/kg
Fuel consumption: 1.0 kg/kg
CO₂ emissions: 0.1 kg CO₂/kg
Material consumption: 1.0 kg/kg
High purity quartz (SiO₂) for PV raw materials
High-purity quartz is the raw material for silicon, which is necessary for the manufacture of solar panels and requires a sophisticated process from ore mining to refining and calcination.
Input power (kWh/kg)
5 – 10 kWh/kg
(crushing, sieving, chemical processing, calcination, drying facilities in operation)
- input fuel
Type: Natural gas, heavy oil
Approximate quantity:
Natural gas: 0.01 – 0.03 kg/kg (used in heating and drying processes)
Heavy oil: 0.005 – 0.01 kg/kg (fuel for firing furnaces)
3 CO₂ emissions other than combustion
0.01 – 0.05 kg CO₂/kg
(emissions from side reactions during chemical processing)
- the amount of change to resources such as earth, rocks, and water
Quartz ore: 1.1 – 1.5 kg/kg (processed to obtain high-purity SiO₂)
Water: 1 – 2 kg/kg (used for cleaning and cooling processes)
- input item name and input amount guideline
Quartz ore: 1.1 – 1.5 kg
Acids (hydrochloric acid, hydrofluoric acid): 0.1 – 0.2 kg
Alkali (sodium hydroxide): 0.05 – 0.1 kg
(for dissolution and purification of impurities)
- process substances such as solvents
Name: Water, pickling solution
Usage: 1 – 2 kg/kg
Circulation rate: 50 – 80% (some cleaning water is reused)
- waste requiring special treatment
Name: Sludge, acidic liquid waste
Approximate quantity: 0.1 – 0.2 kg/kg
(effluent containing impurities must be treated appropriately)
Reference Process and Background
Production process for high-purity quartz: Mined quartz ore is crushed and refined, then chemically treated to remove impurities. After calcination, a high purity of SiO₂ content is ensured.
Environmental Impact and Management: Proper management of acidic effluents generated by chemical processing is required.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 5 – 10 kWh/kg |
| Natural gas consumption | 0.01 – 0.03 kg/kg |
| Fuel Oil Consumption | 0.005 – 0.01 kg/kg |
| CO₂ emissions other than combustion | 0.01 – 0.05 kg CO₂/kg |
| Consumption of quartz ore | 1.1 – 1.5 kg/kg |
| Water usage | 1 – 2 kg/kg |
| Acid and alkali usage | 0.1 – 0.3 kg/kg |
| Waste (sludge and acidic liquid waste) | 0.1 – 0.2 kg/kg |
Alumina (Al₂O₃)
Alumina is refined mainly from bauxite ore by the Bayer process and is used as a raw material for aluminum production.
Input power (kWh/kg)
0.3 – 0.5 kWh/kg
(used to run crushing, separation, filtration, calcination, and drying facilities)
- input fuel
Type: Natural gas, heavy oil
Approximate quantity:
Natural gas: 0.02 – 0.05 kg/kg (used for heating and firing processes)
Heavy oil: 0.01 – 0.03 kg/kg (used as auxiliary fuel)
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.1 – 0.3 kg CO₂/kg
(emissions from chemical reactions during bauxite refining and volatilization from solution)
- amount of change in resources such as soil, stone, and water (kg)
Bauxite ore: 2 – 3 kg/kg
Water: 1 – 3 kg/kg (used for cleaning, filtration, cooling)
- input item name and input amount guideline
Bauxite ore: 2 – 3 kg
Sodium hydroxide (NaOH): 0.05 – 0.1 kg
(for alumina dissolution)
Limestone (CaCO₃): 0.01 – 0.05 kg
(impurity precipitant)
- process substances such as solvents
Name: Water, NaOH solution
Usage: 1 – 3 kg/kg
Circulation rate: 50 – 70% (some water can be reused)
- waste requiring special treatment
Name: Red mud (ore residue), waste liquid
Approximate quantity: 1 – 2 kg/kg
(red mud is alkaline and requires landfill treatment)
Reference Process and Background
In the buyer’s method, bauxite ore is crushed, alumina is eluted with NaOH, impurities are precipitated, and alumina is recovered and calcined.
Environmental Impact: The red mud produced in the manufacturing process is large in volume and must be properly managed.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.3 – 0.5 kWh/kg |
| Natural gas consumption | 0.02 – 0.05 kg/kg |
| Fuel Oil Consumption | 0.01 – 0.03 kg/kg |
| CO₂ emissions other than combustion | 0.1 – 0.3 kg CO₂/kg |
| Bauxite ore consumption | 2 – 3 kg/kg |
| Water usage | 1 – 3 kg/kg |
| Amount of sodium hydroxide used | 0.05 – 0.1 kg/kg |
| Waste (red mud and liquid waste) | 1 – 2 kg/kg |
Carbon electrode for smelting (Graphite electrode)
Carbon electrodes for smelting are mainly made from petroleum coke or tar pitch, and are produced through high-temperature sintering and graphitization.
Input power (kWh/kg)
1.5 – 2.5 kWh/kg
(used for grinding, mixing, forming, sintering, graphitizing, and finishing)
- input fuel
Type: Natural gas, electricity (indirect fuel consumption), heavy oil
Approximate quantity:
Natural gas: 0.05 – 0.1 kg/kg (used in firing process)
Heavy oil: 0.01 – 0.02 kg/kg (for auxiliary heating)
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.1 – 0.3 kg CO₂/kg
(emissions from side reactions during high-temperature calcination and graphitization processes)
- amount of change in resources such as soil, stone, and water (kg)
Petroleum coke: 1.2 – 1.5 kg/kg
Tar pitch: 0.3 – 0.5 kg/kg
Water: 0.2 – 0.5 kg/kg (used for cooling and cleaning processes)
- input item name and input amount guideline
Petroleum coke: 1.2 – 1.5 kg
Tar pitch: 0.3 – 0.5 kg
Catalyst/stabilizer (depending on case): 0.01 – 0.02 kg
- process substances such as solvents
Name: Water
Usage: 0.2 – 0.5 kg/kg
Circulation rate: 50 – 80% (cooling water is reused)
- waste requiring special treatment
Name: Dust, waste tar, waste water
Approximate quantity: 0.05 – 0.1 kg/kg
(dust and waste tar require appropriate treatment)
Reference Process and Background
Manufacturing process: Petroleum coke and tar pitch are mixed and molded, followed by high-temperature sintering and further graphitization at temperatures above 3000℃. This produces carbon electrodes with high electrical conductivity and heat resistance.
Environmental Impact: Exhaust gas treatment during the firing process and proper management of waste tar and wastewater are important.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 1.5 – 2.5 kWh/kg |
| Natural gas consumption | 0.05 – 0.1 kg/kg |
| Fuel Oil Consumption | 0.01 – 0.02 kg/kg |
| CO₂ emissions other than combustion | 0.1 – 0.3 kg CO₂/kg |
| Petroleum coke consumption | 1.2 – 1.5 kg/kg |
| Tar pitch consumption | 0.3 – 0.5 kg/kg |
| Water usage | 0.2 – 0.5 kg/kg |
| Waste (dust and waste tar) | 0.05 – 0.1 kg/kg |
Red mud (bauxite residue) treatment
Red mud is a byproduct of alumina production and is usually managed by neutralization, compression dewatering, or solidification. Treatment is performed in an appropriate manner to minimize environmental impact.
Input power (kWh/kg)
0.05 – 0.15 kWh/kg
(operation of compression, dewatering, and neutralization facilities)
- input fuel
Type: Electricity, natural gas
Approximate quantity:
Natural gas: 0.005 – 0.01 kg/kg (if drying or calcination is required)
CO₂ emissions other than combustion (kg CO₂/kg)
0.01 – 0.03 kg CO₂/kg
(emissions from chemical reactions in neutralization treatment and carbonate use)
- amount of change in resources such as soil, stone, and water (kg)
Water: 1 – 2 kg/kg (used for washing, compression dewatering, cooling)
- input item name and input amount guideline
Neutralizer (e.g. limestone, slaked lime): 0.05 – 0.1 kg/kg
(to neutralize acidic components)
Flocculant (e.g. polymer flocculant): 0.005 – 0.01 kg/kg
(improves efficiency of solid-liquid separation)
- process substances such as solvents
Name: Water
Usage: 1 – 2 kg/kg
Recirculation rate: 50 – 80% (part of the wash water is reused)
- waste requiring special treatment
Name: Concentration residue, liquid waste
Approximate amount: 0.1 – 0.2 kg/kg
(solid residue after treatment is landfilled, effluent is discharged after neutralization treatment)
Reference Process and Background
Treatment method: In the neutralization process, lime or carbonate is added to the red mud to neutralize the acidic components. The mud is then dewatered and compacted, and the solid residue is disposed of as landfill.
Environmental Management: Appropriate management of liquid waste and solid residues is required and will be discharged in accordance with discharge standards.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.05 – 0.15 kWh/kg |
| Natural gas consumption | 0.005 – 0.01 kg/kg |
| CO₂ emissions other than combustion | 0.01 – 0.03 kg CO₂/kg |
| Water usage | 1 – 2 kg/kg |
| Limestone and slaked lime usage | 0.05 – 0.1 kg/kg |
| Coagulant usage | 0.005 – 0.01 kg/kg |
| Waste (concentrate residue and liquid waste) | 0.1 – 0.2 kg/kg |
Treatment of waste carbon electrodes (graphite electrodes, etc.)
The most common ways to dispose of waste carbon electrodes are crushing and recycling, pyrolysis, or use in metal refining.
Input power (kWh/kg)
0.3 – 0.8 kWh/kg
(required for grinding, separation, drying, and preparation for reuse)
- input fuel
Type: Natural gas, heavy oil
Approximate quantity:
Natural gas: 0.01 – 0.05 kg/kg (for thermal processing and drying)
Heavy oil: 0.005 – 0.01 kg/kg (fuel for heating furnaces)
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.05 – 0.1 kg CO₂/kg
(generated by pyrolysis or chemical reprocessing)
- amount of change in resources such as soil, stone, and water (kg)
Water: 0.5 – 1.0 kg/kg
(used for cooling and dust suppression)
- input item name and input amount guideline
Sodium hydroxide (NaOH): 0.01 – 0.05 kg/kg
(used to neutralize acidic components)
Acid (sulfuric acid, hydrochloric acid): 0.01 – 0.03 kg/kg
(used to treat metal content)
- process substances such as solvents
Name: Water, pickling solution
Usage: 0.5 – 1.0 kg/kg
Circulation rate: 50 – 70% (partially reusable)
- waste requiring special treatment
Name: Wastewater, residues (contained metals, dust)
Approximate quantity: 0.05 – 0.1 kg/kg
(metal-containing residues are often recycled)
Reference Process and Background
Reuse Process: Crushed carbon electrodes are generally reused in the foundry industry and metal smelting. The metal content in the spent electrodes is also recovered.
Thermal treatment: Thermal decomposition or incineration at high temperatures is used to remove impurities. Proper control of exhaust gas is required during the treatment process.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.3 – 0.8 kWh/kg |
| Natural gas consumption | 0.01 – 0.05 kg/kg |
| Fuel Oil Consumption | 0.005 – 0.01 kg/kg |
| CO₂ emissions other than combustion | 0.05 – 0.1 kg CO₂/kg |
| Water usage | 0.5 – 1.0 kg/kg |
| Amount of acid and alkali used | 0.01 – 0.05 kg/kg |
| Waste (wastewater and residues) | 0.05 – 0.1 kg/kg |
Quartz sand (SiO₂)
Quartz sand is collected by open-pit mining or suction extraction of river sand and requires processing by washing and sieving. High purity quartz sand is used in glass and silicon manufacturing.
Input power (kWh/kg)
0.02 – 0.05 kWh/kg
(operation of sieving, washing, and conveyor for transport)
- input fuel
Type: Light oil, natural gas
Approximate quantity:
Diesel oil: 0.01 – 0.03 kg/kg (used for excavation and transportation with excavators and dump trucks)
Natural gas: 0.005 – 0.01 kg/kg (used in drying process)
CO₂ emissions other than combustion (kg CO₂/kg)
0.002 – 0.005 kg CO₂/kg
(indirect emissions from dust suppressants, transportation and machine operation)
- amount of change in resources such as soil, stone, and water (kg)
Soil and rocks: 1.1 – 1.3 kg/kg (unwanted rocks and soil from post-mining separation)
Water: 1 – 2 kg/kg (used for cleaning and cooling)
- input item name and input amount guideline
Quartz ore: 1.1 – 1.3 kg (sieved after adjusting the quantity required)
Dust suppressant: 0.001 – 0.005 kg/kg (applied at excavation site)
- process substances such as solvents
Name: Cleaning water
Usage: 1 – 2 kg/kg
Circulation rate: 50 – 70% (some wash water can be reused)
- waste requiring special treatment
Name: Residual soil, wastewater (contains trace amounts of impurities)
Approximate quantity: 0.1 – 0.2 kg/kg (must be landfilled or reused)
Reference Process and Background
Mining Method: Quartz sand is extracted by open-pit mining or river sand suction pumps, and purity is adjusted through sieving and washing.
Environmental Impact: Impurities and residual soil in wastewater must be managed and properly treated.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.02 – 0.05 kWh/kg |
| Diesel oil consumption | 0.01 – 0.03 kg/kg |
| Natural gas consumption | 0.005 – 0.01 kg/kg |
| CO₂ emissions other than combustion | 0.002 – 0.005 kg CO₂/kg |
| Amount of soil and stone removed | 1.1 – 1.3 kg/kg |
| Water usage | 1 – 2 kg/kg |
| Amount of dust suppressant used | 0.001 – 0.005 kg/kg |
| Waste (overburden and wastewater) | 0.1 – 0.2 kg/kg |
Dolomite concentrate (CaMg(CO₃)₂)
Dolomite is used in construction materials, the steelmaking industry, and glass production. It is usually finished as a concentrate through open-pit mining and subsequent crushing and sieving.
Input power (kWh/kg)
0.02 – 0.05 kWh/kg
(operation of conveyors for crushing, sieving, washing and transport)
- input fuel
Type: Light oil, natural gas
Approximate quantity:
Diesel oil: 0.01 – 0.03 kg/kg (fuel for mining equipment and dump trucks)
Natural gas: 0.005 – 0.01 kg/kg (if used in drying or firing processes)
CO₂ emissions other than combustion (kg CO₂/kg)
0.01 – 0.05 kg CO₂/kg
(emissions from grinding and transport, volatile reactions of carbonates)
- amount of change in resources such as soil, stone, and water (kg)
Soil and stone: 1.2 – 1.5 kg/kg (soil and stone removed in ore concentrate)
Water: 0.5 – 1.0 kg/kg (for dust control and cleaning)
- input item name and input amount guideline
Dolomite ore: 1.2 – 1.5 kg/kg (processed as concentrate)
Dust suppressant: 0.001 – 0.005 kg/kg (used at mining sites)
- process substances such as solvents
Name: Water
Usage: 0.5 – 1.0 kg/kg
Recirculation rate: 50 – 80% (part of the wash water is reused)
- waste requiring special treatment
Name: Residual soil, wastewater (including impurities)
Approximate quantity: 0.1 – 0.2 kg/kg (must be landfilled or reused)
Reference Process and Background
Mining Method: Dolomite ore is crushed and sieved after open-pit mining to the appropriate size.
Environmental management: Dust control and wastewater treatment are required, and waste is managed according to regulations.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.02 – 0.05 kWh/kg |
| Diesel oil consumption | 0.01 – 0.03 kg/kg |
| Natural gas consumption | 0.005 – 0.01 kg/kg |
| CO₂ emissions other than combustion | 0.01 – 0.05 kg CO₂/kg |
| Amount of soil and stone removed | 1.2 – 1.5 kg/kg |
| Water usage | 0.5 – 1.0 kg/kg |
| Amount of dust suppressant used | 0.001 – 0.005 kg/kg |
| Waste (overburden and wastewater) | 0.1 – 0.2 kg/kg |
Copper concentrate (Cu concentrate)
Copper concentrates are obtained by crushing copper sulfide ores (e.g., brass ores, pyrite) and concentrating the copper content by flotation beneficiation or other methods.
Input power (kWh/kg)
0.1 – 0.3 kWh/kg
(crushing, flotation beneficiation, dewatering, conveyor operation)
- input fuel
Type: Diesel oil, electricity (indirect fuel), natural gas
Approximate quantity:
Diesel oil: 0.02 – 0.05 kg/kg (fuel for mining and transportation vehicles)
Natural gas: 0.005 – 0.01 kg/kg (for heating drying furnaces)
CO₂ emissions other than combustion (kg CO₂/kg)
0.05 – 0.1 kg CO₂/kg
(secondary emissions from beneficiation and chemical use)
- amount of change in resources such as soil, stone, and water (kg)
Ore: 20 – 30 kg/kg (0.5 – 2.0% grade copper ore)
Water: 3 – 5 kg/kg (for ore dressing and dust suppression)
- input item name and input amount guideline
Copper sulfide ore: 20 – 30 kg
Chemicals (sorbents, foaming agents): 0.01 – 0.05 kg/kg
(used in ore dressing)
- process substances such as solvents
Name: Water, flotation beneficiation agent
Usage: 3 – 5 kg/kg
Circulation rate: 60 – 80% (reused in the beneficiation process)
- waste requiring special treatment
Name: Tailings, drainage
Approximate quantity: 15 – 25 kg/kg (tailings need to be reclaimed or reused)
Reference Process and Background
In the flotation beneficiation process, the ore is crushed and the copper minerals are separated using froth and chemicals. Tailings are generated in large quantities and are important to control.
Environmental management: Tailings and wastewater are properly treated according to environmental standards, and wastewater is partially reused after purification.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.1 – 0.3 kWh/kg |
| Diesel oil consumption | 0.02 – 0.05 kg/kg |
| Natural gas consumption | 0.005 – 0.01 kg/kg |
| CO₂ emissions other than combustion | 0.05 – 0.1 kg CO₂/kg |
| Ore consumption | 20 – 30 kg/kg |
| Water usage | 3 – 5 kg/kg |
| Chemical usage | 0.01 – 0.05 kg/kg |
| Waste (tailings and wastewater) | 15 – 25 kg/kg |
glass fiber
Glass fiber is produced through a process in which glass, consisting mainly of silica (quartz sand), soda ash, and limestone, is melted and converted into fiber.
Input power (kWh/kg)
1.0 – 2.0 kWh/kg
(electric furnace, molten glass processing, drawing and finishing processes)
- input fuel
Type: Natural gas, heavy oil
Approximate quantity:
Natural gas: 0.3 – 0.5 kg/kg (for melting furnace heating)
Heavy oil: 0.01 – 0.05 kg/kg (used as auxiliary fuel)
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.1 – 0.2 kg CO₂/kg
(emissions from decomposition and chemical reaction of carbonate materials)
- amount of change in resources such as soil, stone, and water (kg)
Quartz sand (SiO₂): 0.5 – 0.6 kg/kg
Soda ash (Na₂CO₃): 0.1 – 0.2 kg/kg
Limestone (CaCO₃): 0.05 – 0.1 kg/kg
Water: 1 – 3 kg/kg (used for cooling and cleaning)
- input item name and input amount guideline
Quartz sand: 0.5 – 0.6 kg
Soda ash: 0.1 – 0.2 kg
Limestone: 0.05 – 0.1 kg
Borax (B₂O₃, if used): 0.01 – 0.05 kg
Binder: 0.01 – 0.03 kg (for forming and holding fibers)
- process substances such as solvents
Name: Water
Usage: 1 – 3 kg/kg
Circulation rate: 50 – 80% (cooling and cleaning water reuse)
- waste requiring special treatment
Name: Dust, wastewater, glass waste
Approximate quantity: 0.05 – 0.1 kg/kg
(dust and glass waste are often recycled)
Reference Process and Background
Manufacturing process: Quartz sand, soda ash, limestone, etc. are melted in a furnace and stretched into thin glass threads to form fiber. Then, cooling and surface treatment are performed.
Environmental Management: Appropriate management of wastewater treatment and exhaust gas is required. Glass waste is generally re-melted and reused.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 1.0 – 2.0 kWh/kg |
| Natural gas consumption | 0.3 – 0.5 kg/kg |
| Fuel Oil Consumption | 0.01 – 0.05 kg/kg |
| CO₂ emissions other than combustion | 0.1 – 0.2 kg CO₂/kg |
| Amount of quartz sand used | 0.5 – 0.6 kg/kg |
| Amount of soda ash used | 0.1 – 0.2 kg/kg |
| Amount of limestone used | 0.05 – 0.1 kg/kg |
| Water usage | 1 – 3 kg/kg |
| Waste (dust and glass waste) | 0.05 – 0.1 kg/kg |
epoxy resin
Epoxy resins are mainly composed of bisphenol A (BPA) and epichlorohydrin (ECH) and are produced through a polymerization reaction.
Input power (kWh/kg)
1.0 – 2.5 kWh/kg
(polymerization reaction agitation, temperature control, purifier operation)
- input fuel
Type: Electricity, natural gas
Approximate quantity:
Natural gas: 0.01 – 0.03 kg/kg (heating and drying process)
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.2 – 0.5 kg CO₂/kg
(emissions from reactions and solvent use)
- amount of change in resources such as soil, stone, and water (kg)
Water: 2 – 5 kg/kg (cooling, cleaning)
- input item name and input amount guideline
Bisphenol A (BPA): 0.6 – 0.7 kg
Epichlorohydrin (ECH): 0.3 – 0.4 kg
Sodium hydroxide (NaOH): 0.05 – 0.1 kg
(for treatment of by-products)
- process substances such as solvents
Name: Organic solvent (water, ethanol, acetone)
Usage: 0.2 – 0.5 kg/kg
Recirculation rate: 60 – 80% (part of the solvent can be reused)
- waste requiring special treatment
Name: Wastewater, spent solvents, solid wastes (byproducts of byproduct reactions)
Approximate quantity: 0.1 – 0.3 kg/kg
(requires proper treatment and disposal)
Reference Process and Background
Manufacturing process: BPA reacts with ECH to produce epoxy resin monomer. Polymerization is then carried out under temperature control. Cooling and purification are critical, and the by-products generated must be controlled.
Environmental Impact: Emission control of liquid waste and volatile organic compounds (VOCs) is required.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 1.0 – 2.5 kWh/kg |
| Natural gas consumption | 0.01 – 0.03 kg/kg |
| CO₂ emissions other than combustion | 0.2 – 0.5 kg CO₂/kg |
| Water usage | 2 – 5 kg/kg |
| Bisphenol A usage | 0.6 – 0.7 kg/kg |
| Amount of epichlorohydrin used | 0.3 – 0.4 kg/kg |
| Amount of sodium hydroxide used | 0.05 – 0.1 kg/kg |
| Waste (wastewater and solvents) | 0.1 – 0.3 kg/kg |
Curing agent (e.g., polyamine-based, polyamide-based, etc.)
Curing agents promote chemical reactions in polymer materials such as epoxy resins to improve their mechanical and chemical performance.
Input power (kWh/kg)
0.8 – 2.0 kWh/kg
(used in reaction, temperature control, stirring, drying, and refining processes)
- input fuel
Type: Natural gas, heavy oil
Approximate quantity:
Natural gas: 0.01 – 0.03 kg/kg (used for heating and drying processes)
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.1 – 0.3 kg CO₂/kg
(due to chemical reactions and emissions of volatile organic compounds (VOCs))
- amount of change in resources such as soil, stone, and water (kg)
Water: 1 – 2 kg/kg (for cooling and cleaning)
- input item name and input amount guideline
Raw amines (e.g. ethylenediamine, triethylenetetetramine): 0.6 – 0.8 kg
Fatty acids (e.g. ricinoleic acid): 0.1 – 0.2 kg
Stabilizer/accelerator: 0.01 – 0.03 kg
- process substances such as solvents
Name: Organic solvent (e.g., methanol, ethanol)
Usage: 0.1 – 0.3 kg/kg
Recirculation rate: 60 – 80% (some solvents are distilled and reused)
- waste requiring special treatment
Name: Waste solvents, reaction byproducts, wastewater
Approximate quantity: 0.1 – 0.2 kg/kg (proper handling required)
Reference Process and Background
Manufacturing process: Reaction of raw material amines with fatty acids to produce a curing agent at an appropriate and controlled temperature. Environmental control is required to reduce emissions of volatile organic compounds (VOCs).
Environmental impact: Proper treatment of spent solvents and wastewater is required, and waste management is important.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.8 – 2.0 kWh/kg |
| Natural gas consumption | 0.01 – 0.03 kg/kg |
| CO₂ emissions other than combustion | 0.1 – 0.3 kg CO₂/kg |
| Water usage | 1 – 2 kg/kg |
| Amount of amines used | 0.6 – 0.8 kg/kg |
| Fatty acid usage | 0.1 – 0.2 kg/kg |
| Solvent usage | 0.1 – 0.3 kg/kg |
| Waste (waste solvents and wastewater) | 0.1 – 0.2 kg/kg |
Carbon fiber for reinforcement (e.g., PAN-based carbon fiber)
Carbon fiber is made primarily from polyacrylonitrile (PAN) and is manufactured through oxidation, carbonization, graphitization, and other high-temperature processes.
Input power (kWh/kg)
100 – 200 kWh/kg
(operation of carbonizing furnaces, oxidizing furnaces, graphite furnaces, and processing processes such as winding and cutting)
- input fuel
Type: Natural gas, heavy oil
Approximate quantity:
Natural gas: 0.1 – 0.2 kg/kg (used to heat the carbonization and calcination processes)
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.5 – 1.0 kg CO₂/kg
(volatile components in the carbon fiber conversion process from PAN)
- amount of change in resources such as soil, stone, and water (kg)
Water: 2 – 5 kg/kg (used for cooling and cleaning)
- input item name and input amount guideline
PAN raw material (polyacrylonitrile): 1.5 – 2.0 kg/kg (precursor for obtaining fiber)
Oxidation accelerator: 0.01 – 0.05 kg/kg (used in oxidation process)
- process substances such as solvents
Name: Water, acidic or alkaline cleaning solution
Usage: 2 – 5 kg/kg
Circulation rate: 50 – 80% (reuse of cooling and cleaning water)
- waste requiring special treatment
Name: Wastewater, gas (volatile components), PAN residue
Approximate quantity: 0.1 – 0.3 kg/kg (wastewater requires purification, gas requires flue gas treatment)
Reference Process and Background
Manufacturing process: PAN fibers are stabilized in an oxidation furnace and treated at high temperatures in a carbonization furnace. The final graphitization improves strength.
Environmental management: Exhaust gas treatment and control of volatile compounds are required. Reuse of used water and solvents is also recommended.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 100 – 200 kWh/kg |
| Natural gas consumption | 0.1 – 0.2 kg/kg |
| CO₂ emissions other than combustion | 0.5 – 1.0 kg CO₂/kg |
| Water usage | 2 – 5 kg/kg |
| Amount of PAN raw materials used | 1.5 – 2.0 kg/kg |
| Amount of oxidation accelerator used | 0.01 – 0.05 kg/kg |
| Waste (wastewater and gas) | 0.1 – 0.3 kg/kg |
light oil
Diesel oil is the middle distillate obtained in the crude oil refining process and is produced through distillation, cracking, refining, and desulfurization.
Input power (kWh/kg)
0.1 – 0.3 kWh/kg
(distillation column, furnace, pumps, FGD unit in operation)
- input fuel
Type: Natural gas, heavy oil
Approximate quantity:
Natural gas: 0.02 – 0.05 kg/kg (for heating process and hydrogen production)
Heavy oil: 0.01 – 0.03 kg/kg (as auxiliary fuel)
CO₂ emissions other than combustion (kg CO₂/kg)
0.01 – 0.05 kg CO₂/kg
(secondary emissions from hydrogen desulfurization process and chemical reactions)
- amount of change in resources such as soil, stone, and water (kg)
Crude oil: 1.2 – 1.5 kg/kg (adjusted according to yield in the production process)
Water: 1 – 2 kg/kg (used for cooling and processing)
- input item name and input amount guideline
Crude oil: 1.2 – 1.5 kg
Hydrogen gas: 0.01 – 0.05 kg (used for desulfurization process)
Catalyst: 0.005 – 0.01 kg/kg (for desulfurization and refining)
- process substances such as solvents
Name: Water, chemicals (caustic soda, etc.)
Usage: 1 – 2 kg/kg
Circulation rate: 50 – 80% (reuse of cooling water and cleaning water)
- waste requiring special treatment
Name: Waste catalyst, oxide residue, wastewater
Approximate quantity: 0.05 – 0.1 kg/kg (requires appropriate treatment)
Reference Process and Background
Manufacturing Process: Crude oil is distilled to extract the light oil fraction, which is then desulfurized or otherwise treated. In most cases, pressurized desulfurization using hydrogen gas is used.
Environmental management: It is important to control wastewater and waste catalyst in the manufacturing process.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.1 – 0.3 kWh/kg |
| Natural gas consumption | 0.02 – 0.05 kg/kg |
| Fuel Oil Consumption | 0.01 – 0.03 kg/kg |
| CO₂ emissions other than combustion | 0.01 – 0.05 kg CO₂/kg |
| Crude oil usage | 1.2 – 1.5 kg/kg |
| Water usage | 1 – 2 kg/kg |
| Amount of hydrogen gas used | 0.01 – 0.05 kg/kg |
| Waste (waste catalyst and waste water) | 0.05 – 0.1 kg/kg |
Polycarboxylic acid ether (PCE)
PCE is mainly used as a water reducer in concrete, etc. In the manufacturing process, it is synthesized from ethylene glycol and methacrylic acid in a polymerization reaction.
Input power (kWh/kg)
0.5 – 1.5 kWh/kg
(used to run agitators, reaction vessels, temperature control, filtration and drying equipment)
- input fuel
Type: Natural gas, heavy oil
Approximate quantity:
Natural gas: 0.01 – 0.02 kg/kg (used for heating process)
CO₂ emissions other than combustion (kg CO₂/kg)
0.1 – 0.3 kg CO₂/kg
(emitted during chemical reaction and treatment of by-products)
- amount of change in resources such as soil, stone, and water (kg)
Water: 2 – 4 kg/kg (for cooling and cleaning)
- input item name and input amount guideline
Methacrylic acid: 0.2 – 0.4 kg/kg
Ethylene glycol: 0.5 – 0.7 kg/kg
Initiator (e.g., benzoyl peroxide): 0.01 – 0.05 kg/kg
Catalyst: 0.005 – 0.01 kg/kg (promotes polymerization reaction)
- process substances such as solvents
Name: Water, organic solvent (ethanol, etc.)
Usage: 0.5 – 1.5 kg/kg
Circulation rate: 50 – 80% (cooling water and solvent reuse)
- waste requiring special treatment
Name: Reaction byproducts, liquid waste, spent solvents
Approximate quantity: 0.1 – 0.3 kg/kg
(effluent is disposed of properly, solvents are reused whenever possible)
Reference Process and Background
Production Process: PCE is synthesized by polymerization of methacrylic acid and ethylene glycol. The reaction takes place in a reactor with controlled temperature and agitation, and the by-products are filtered and dried.
Environmental management: Appropriate treatment of waste liquids and used solvents is required. VOC (Volatile Organic Compounds) control is required for emissions.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.5 – 1.5 kWh/kg |
| Natural gas consumption | 0.01 – 0.02 kg/kg |
| CO₂ emissions other than combustion | 0.1 – 0.3 kg CO₂/kg |
| Water usage | 2 – 4 kg/kg |
| Amount of methacrylic acid used | 0.2 – 0.4 kg/kg |
| Amount of ethylene glycol used | 0.5 – 0.7 kg/kg |
| Catalyst and initiator usage | 0.01 – 0.05 kg/kg |
| Waste (effluents and byproducts) | 0.1 – 0.3 kg/kg |
Preservatives (e.g., parabens, isothiazolinones, formaldehyde)
Production involves chemical reactions, mixing, and purification processes.
Input power (kWh/kg)
0.5 – 1.5 kWh/kg
(used for agitation of reaction vessel, temperature control, and operation of drying and filtration equipment)
- input fuel
Type: Natural gas, electricity (indirect use)
Approximate quantity:
Natural gas: 0.01 – 0.02 kg/kg (used to heat the drying process)
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.1 – 0.2 kg CO₂/kg
(CO₂ emissions from chemical reactions and side reactions)
- amount of change in resources such as soil, stone, and water (kg)
Water: 1 – 2 kg/kg (for cooling and cleaning)
- input item name and input amount guideline
Phenolic compounds (e.g. parabens): 0.3 – 0.5 kg
Acid (e.g. acetic acid): 0.1 – 0.2 kg
Alcohol (e.g. ethanol): 0.2 – 0.3 kg
Catalyst/stabilizer: 0.01 – 0.05 kg
- process substances such as solvents
Name: Organic solvent (ethanol, water)
Usage: 0.5 – 1.0 kg/kg
Circulation rate: 60 – 80% (solvents are often reused)
- waste requiring special treatment
Name: Waste liquids, spent solvents, byproducts
Approximate quantity: 0.1 – 0.2 kg/kg
(requires proper disposal or recycling)
Reference Process and Background
Manufacturing process: Produced by reacting organic acids, alcohols, and phenolic compounds. Temperature and agitation control is important, and appropriate treatment of byproducts is required.
Environmental management: Reuse of used solvents and treatment of organic matter in wastewater are required.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.5 – 1.5 kWh/kg |
| Natural gas consumption | 0.01 – 0.02 kg/kg |
| CO₂ emissions other than combustion | 0.1 – 0.2 kg CO₂/kg |
| Water usage | 1 – 2 kg/kg |
| Amount of phenol compound used | 0.3 – 0.5 kg/kg |
| Acid usage | 0.1 – 0.2 kg/kg |
| Alcohol usage | 0.2 – 0.3 kg/kg |
| Catalyst/stabilizer usage | 0.01 – 0.05 kg/kg |
| Waste (effluents and byproducts) | 0.1 – 0.2 kg/kg |
Palladium chloride (PdCl₂)
PdCl₂ is produced mainly by the oxidation and chloride reaction of palladium metal and is used in the production of catalysts and electronic materials.
Input power (kWh/kg)
2.0 – 4.0 kWh/kg
(used for reaction control, stirrer, filtration, drying)
- input fuel
Type: Natural gas, electricity (indirect use)
Approximate quantity:
Natural gas: 0.01 – 0.03 kg/kg (for heating and drying process)
CO₂ emissions other than combustion (kg CO₂/kg)
0.05 – 0.1 kg CO₂/kg
(emissions from chemical reaction by-products and solvent use)
- amount of change in resources such as soil, stone, and water (kg)
Water: 1 – 2 kg/kg (washing and cooling)
- input item name and input amount guideline
Palladium metal (Pd): 0.6 – 0.7 kg
Hydrochloric acid (HCl): 0.3 – 0.5 kg (for chloride process)
Oxidant (e.g., nitric acid): 0.05 – 0.1 kg (to accelerate the oxidation process)
- process substances such as solvents
Name: Water, hydrochloric acid solution
Usage: 1 – 2 kg/kg
Circulation rate: 50 – 70% (solvent reuse)
- waste requiring special treatment
Name: Waste acid, metal residue, wastewater
Approximate quantity: 0.1 – 0.2 kg/kg (waste acid is reused or neutralized)
Reference Process and Background
Manufacturing process: Palladium metal is reacted with hydrochloric acid to produce palladium chloride, which may also be used as an oxidizing agent. The solution is then filtered and dried to obtain the final product.
Environmental Management: Proper treatment of waste acid and metal residues is required. Water reuse and wastewater treatment are also required.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 2.0 – 4.0 kWh/kg |
| Natural gas consumption | 0.01 – 0.03 kg/kg |
| CO₂ emissions other than combustion | 0.05 – 0.1 kg CO₂/kg |
| Water usage | 1 – 2 kg/kg |
| Palladium metal usage | 0.6 – 0.7 kg/kg |
| Hydrochloric acid usage | 0.3 – 0.5 kg/kg |
| Amount of oxidizer used | 0.05 – 0.1 kg/kg |
| Waste (waste acid and residue) | 0.1 – 0.2 kg/kg |
γ-Alumina (γ-Al₂O₃)
γ-Alumina is obtained by heating and sintering aluminum hydroxide (Al(OH)₃) produced by the Byers process, and is used as an adsorbent or catalyst support.
Input power (kWh/kg)
0.8 – 1.5 kWh/kg
(drying, kiln temperature control, grinding and finishing processes)
- input fuel
Type: Natural gas, heavy oil
Approximate quantity:
Natural gas: 0.1 – 0.2 kg/kg (for heating firing furnaces)
Heavy oil: 0.01 – 0.03 kg/kg (used as auxiliary fuel)
CO₂ emissions other than combustion (kg CO₂/kg)
0.05 – 0.1 kg CO₂/kg
(emissions during chemical reactions and processes)
- amount of change in resources such as soil, stone, and water (kg)
Water: 1 – 2 kg/kg (used for cooling and dust suppression)
- input item name and input amount guideline
Aluminum hydroxide (Al(OH)₃): 1.8 – 2.2 kg/kg (raw material for γ-alumina)
Acid or alkali (used as regulator): 0.01 – 0.05 kg/kg
- process substances such as solvents
Name: Water
Usage: 1 – 2 kg/kg
Circulation rate: 50 – 70% (cooling water is partially reused)
- waste requiring special treatment
Name: Wastewater, dust, baking residue
Approximate quantity: 0.1 – 0.2 kg/kg (proper handling required)
Reference Process and Background
Manufacturing process: Al(OH)₃ is dried and pyrolyzed in a calcination furnace at 300-500°C to obtain γ-alumina. Appropriate control of calcination temperature is necessary. Particle size control of the product and dust control are also important.
Environmental management: Appropriate control of dust and wastewater generated in the firing process is required.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.8 – 1.5 kWh/kg |
| Natural gas consumption | 0.1 – 0.2 kg/kg |
| Fuel Oil Consumption | 0.01 – 0.03 kg/kg |
| CO₂ emissions other than combustion | 0.05 – 0.1 kg CO₂/kg |
| Water usage | 1 – 2 kg/kg |
| Amount of Al(OH)₃ used | 1.8 – 2.2 kg/kg |
| Acid or alkali usage | 0.01 – 0.05 kg/kg |
| Waste (dust and wastewater) | 0.1 – 0.2 kg/kg |
Kerosene
Kerosene is obtained by refining crude oil and is produced through distillation of middle distillates, desulfurization treatment, and drying.
Input power (kWh/kg)
0.1 – 0.2 kWh/kg
(operation of distillation columns, pumps, desulfurization and drying facilities)
- input fuel
Type: Natural gas, heavy oil
Approximate quantity:
Natural gas: 0.02 – 0.05 kg/kg (used for heating and desulfurization process)
Heavy oil: 0.01 – 0.03 kg/kg (used as auxiliary fuel)
CO₂ emissions other than combustion (kg CO₂/kg)
0.01 – 0.03 kg CO₂/kg
(emissions from chemical reactions and by-products)
- amount of change in resources such as soil, stone, and water (kg)
Crude oil: 1.2 – 1.4 kg/kg (yield adjustment during refining process)
Water: 1 – 2 kg/kg (for cooling and cleaning)
- input item name and input amount guideline
Crude oil: 1.2 – 1.4 kg
Hydrogen gas: 0.01 – 0.03 kg/kg (for desulfurization process)
Catalyst: 0.005 – 0.01 kg/kg (desulfurization catalyst)
- process substances such as solvents
Name: Water, chemicals (caustic soda, etc.)
Usage: 1 – 2 kg/kg
Circulation rate: 60 – 80% (cooling water reuse)
- waste requiring special treatment
Name: Waste catalyst, waste water, oxide residue
Approximate quantity: 0.05 – 0.1 kg/kg (requires appropriate treatment)
Reference Process and Background
Manufacturing Process: Kerosene is produced by desulfurizing and refining the fractions obtained in the crude oil distillation process and adjusting the quality. Desulfurization requires hydrogen gas and a catalyst.
Environmental Management: Management of wastewater and waste catalyst is important and must be reused or disposed of according to disposal standards.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.1 – 0.2 kWh/kg |
| Natural gas consumption | 0.02 – 0.05 kg/kg |
| Fuel Oil Consumption | 0.01 – 0.03 kg/kg |
| CO₂ emissions other than combustion | 0.01 – 0.03 kg CO₂/kg |
| Crude oil usage | 1.2 – 1.4 kg/kg |
| Water usage | 1 – 2 kg/kg |
| Amount of hydrogen gas used | 0.01 – 0.03 kg/kg |
| Catalyst usage | 0.005 – 0.01 kg/kg |
| Waste (waste catalyst and waste water) | 0.05 – 0.1 kg/kg |
Hydrogen fluoride (HF)
Hydrogen fluoride is produced mainly by reacting fluorospar (CaF₂) with sulfuric acid (H₂SO₄)**.
Input power (kWh/kg)
0.5 – 1.0 kWh/kg
(reaction vessel stirring, temperature control, drying and filtration processes)
- input fuel
Type: Natural gas, electricity (indirect use)
Approximate quantity:
Natural gas: 0.01 – 0.03 kg/kg (reaction heating and drying process)
CO₂ emissions other than combustion (kg CO₂/kg)
0.05 – 0.1 kg CO₂/kg
(emissions of by-products of chemical reactions)
- amount of change in resources such as soil, stone, and water (kg)
Water: 0.5 – 1.0 kg/kg (for cooling and cleaning)
- input item name and input amount guideline
Fluorospar (CaF₂): 1.5 – 1.8 kg/kg
Sulfuric acid (H₂SO₄): 0.8 – 1.0 kg/kg
- process substances such as solvents
Name: Water
Usage: 0.5 – 1.0 kg/kg
Circulation rate: 50 – 80% (cooling and cleaning water reuse)
- waste requiring special treatment
Name: Calcium sulfate (CaSO₄, gypsum), wastewater
Approximate quantity: 1.0 – 1.5 kg/kg
(waste is often properly treated and reused)
Reference Process and Background
Manufacturing process: Fluorospar and concentrated sulfuric acid are heated to react, and the resulting hydrogen fluoride gas is condensed and liquefied to make the product. Calcium sulfate (gypsum)** is generated as a byproduct.
Environmental Management: By-product gypsum may be reused as building material or fertilizer, but proper disposal is required.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.5 – 1.0 kWh/kg |
| Natural gas consumption | 0.01 – 0.03 kg/kg |
| CO₂ emissions other than combustion | 0.05 – 0.1 kg CO₂/kg |
| Water usage | 0.5 – 1.0 kg/kg |
| Fluorospar Usage | 1.5 – 1.8 kg/kg |
| Sulfuric Acid Usage | 0.8 – 1.0 kg/kg |
| Waste (CaSO₄, wastewater) | 1.0 – 1.5 kg/kg |
Potassium fluoride (KF)
Potassium fluoride is produced by reacting hydrogen fluoride (HF) with potassium hydroxide (KOH) and is used in catalysts and fluorinating reagents.
Input power (kWh/kg)
0.8 – 1.5 kWh/kg
(used to run agitation, temperature control, filtration, and drying equipment)
- input fuel
Type: Natural gas, heavy oil
Approximate quantity:
Natural gas: 0.01 – 0.02 kg/kg (used for drying and heating processes)
CO₂ emissions other than combustion (kg CO₂/kg)
0.05 – 0.1 kg CO₂/kg
(chemical reactions and by-product emissions)
- amount of change in resources such as soil, stone, and water (kg)
Water: 1 – 2 kg/kg (for cleaning and cooling)
- input item name and input amount guideline
Hydrogen fluoride (HF): 0.6 – 0.8 kg/kg
Potassium hydroxide (KOH): 0.4 – 0.5 kg/kg
- process substances such as solvents
Name: Water
Usage: 1 – 2 kg/kg
Circulation rate: 50 – 70% (some cooling water is reused)
- waste requiring special treatment
Name: Waste liquids, residues (unreacted materials)
Approximate quantity: 0.1 – 0.2 kg/kg (proper handling required)
Reference Process and Background
Manufacturing process: HF and KOH react in aqueous solution to produce KF. The product is filtered and dried before commercialization. Temperature control during the reaction is important, and control of unreacted materials and effluents is also required.
Environmental management: Appropriate treatment of fluoride in effluent is required.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.8 – 1.5 kWh/kg |
| Natural gas consumption | 0.01 – 0.02 kg/kg |
| CO₂ emissions other than combustion | 0.05 – 0.1 kg CO₂/kg |
| Water usage | 1 – 2 kg/kg |
| Amount of hydrogen fluoride used | 0.6 – 0.8 kg/kg |
| Amount of potassium hydroxide used | 0.4 – 0.5 kg/kg |
| Waste (liquid waste and residue) | 0.1 – 0.2 kg/kg |
Fluorine-containing residual liquid treatment
Treatment of fluorine-containing residual liquids usually includes neutralization, precipitation, and evaporation/concentration, and must be properly controlled to meet environmental standards.
Input power (kWh/kg)
0.5 – 1.5 kWh/kg
(operation of agitators, pumps, heating, evaporators/concentrators, etc.)
- input fuel
Type: Natural gas, electricity (indirect use)
Approximate quantity:
Natural gas: 0.01 – 0.03 kg/kg (heating and evaporation processes)
CO₂ emissions other than combustion (kg CO₂/kg)
0.02 – 0.05 kg CO₂/kg
(emissions from chemical reactions and treatment of by-products)
- amount of change in resources such as soil, stone, and water (kg)
Water: 1 – 2 kg/kg (used for cleaning and cooling)
- input item name and input amount guideline
Neutralizer (e.g., calcium hydroxide Ca(OH)₂): 0.1 – 0.2 kg/kg
Flocculant (e.g. polymer flocculant): 0.01 – 0.05 kg/kg
Acid (for adjustment if needed): 0.01 – 0.03 kg/kg
- process substances such as solvents
Name: Water
Usage: 1 – 2 kg/kg
Circulation rate: 50 – 70% (cooling and cleaning water reuse)
- waste requiring special treatment
Name: Precipitate (calcium fluoride CaF₂), waste liquid
Approximate quantity: 0.2 – 0.5 kg/kg (must be landfilled or reused)
Reference Process and Background
Treatment Process: Fluorine-containing residual liquid is first treated with a neutralizing agent such as calcium hydroxide to precipitate as **Calcium Fluoride (CaF₂)**. The residual liquid is then treated by filtration and evaporated and concentrated if necessary.
Environmental Management: The calcium fluoride produced requires appropriate treatment and is managed to meet effluent discharge standards.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.5 – 1.5 kWh/kg |
| Natural gas consumption | 0.01 – 0.03 kg/kg |
| CO₂ emissions other than combustion | 0.02 – 0.05 kg CO₂/kg |
| Water usage | 1 – 2 kg/kg |
| Amount of neutralizer used | 0.1 – 0.2 kg/kg |
| Amount of coagulant used | 0.01 – 0.05 kg/kg |
| Waste (CaF₂ precipitate and liquid waste) | 0.2 – 0.5 kg/kg |
Quartz ore concentrate
Quartz ore is widely used as a raw material for construction materials, glass, and semiconductors, and is refined through ore dressing, washing, and sieving.
Input power (kWh/kg)
0.2 – 0.5 kWh/kg
(crushing, sieving, washing, conveyor operation for transport)
- input fuel
Type: Light oil, natural gas
Approximate quantity:
Diesel oil: 0.02 – 0.05 kg/kg (fuel for excavators and dump trucks)
Natural gas: 0.01 – 0.02 kg/kg (used for drying process)
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.01 – 0.03 kg CO₂/kg
(emissions from chemical processes during mining and grinding)
- amount of change in resources such as soil, stone, and water (kg)
Soil and stone: 2 – 3 kg/kg (removed as impurities)
Water: 1 – 2 kg/kg (used for cleaning and cooling)
- input item name and input amount guideline
Quartz ore: 2 – 3 kg (raw material for 1 kg of concentrate)
Dust suppressant: 0.001 – 0.005 kg/kg (applied at the mining site)
- process substances such as solvents
Name: Water
Usage: 1 – 2 kg/kg
Circulation rate: 60 – 80% (cooling and cleaning water reuse)
- waste requiring special treatment
Name: Impurities, wastewater (including muddy water)
Approximate quantity: 1.0 – 2.0 kg/kg (must be landfilled or reused)
Reference Process and Background
Manufacturing Process: Mined quartz ore is crushed, washed, and sieved to concentrate. Particularly thorough ore dressing is necessary to obtain high-purity quartz.
Environmental management: Wastewater treatment and dust control measures are required. Residual soil is reclaimed or reused as road materials, etc.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.2 – 0.5 kWh/kg |
| Diesel oil consumption | 0.02 – 0.05 kg/kg |
| Natural gas consumption | 0.01 – 0.02 kg/kg |
| CO₂ emissions other than combustion | 0.01 – 0.03 kg CO₂/kg |
| Amount of soil and stone removed | 2 – 3 kg/kg |
| Water usage | 1 – 2 kg/kg |
| Amount of dust suppressant used | 0.001 – 0.005 kg/kg |
| Waste (impurities and wastewater) | 1.0 – 2.0 kg/kg |
Hydrofluoric acid (HF acid)
Hydrofluoric acid is produced primarily by the reaction of fluorospar (CaF₂) with sulfuric acid (H₂SO₄).
Input power (kWh/kg)
0.8 – 1.5 kWh/kg
(stirring, heating reactor, filtration and evaporation/condensation process)
- input fuel
Type: Natural gas, electricity (indirect use)
Approximate quantity:
Natural gas: 0.02 – 0.05 kg/kg (heating and drying process)
CO₂ emissions other than combustion (kg CO₂/kg)
0.05 – 0.1 kg CO₂/kg
(emissions from by-products during the reaction process)
- amount of change in resources such as soil, stone, and water (kg)
Water: 1 – 2 kg/kg (for cooling and cleaning)
- input item name and input amount guideline
Fluorospar (CaF₂): 1.5 – 1.8 kg
Sulfuric acid (H₂SO₄): 0.8 – 1.0 kg
- process substances such as solvents
Name: Water
Usage: 1 – 2 kg/kg
Circulation rate: 50 – 80% (cooling and cleaning water reuse)
- waste requiring special treatment
Name: Calcium sulfate (CaSO₄), wastewater
Approximate quantity: 1.0 – 1.5 kg/kg (gypsum can be landfilled or reused)
Reference Process and Background
Production process: CaF₂ and H₂SO₄ react under high temperature to produce hydrofluoric acid. Calcium sulfate (gypsum) is produced as a byproduct. The generated hydrogen fluoride gas is condensed and commercialized as an acidic aqueous solution.
Environmental Management: Waste gypsum may be reused as a building material, but proper management of wastewater treatment is required.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.8 – 1.5 kWh/kg |
| Natural gas consumption | 0.02 – 0.05 kg/kg |
| CO₂ emissions other than combustion | 0.05 – 0.1 kg CO₂/kg |
| Water usage | 1 – 2 kg/kg |
| Fluorospar Usage | 1.5 – 1.8 kg/kg |
| Sulfuric Acid Usage | 0.8 – 1.0 kg/kg |
| Waste (gypsum, wastewater) | 1.0 – 1.5 kg/kg |
Petroleum Coke
Petroleum coke is produced as a byproduct of thermal or catalytic cracking of heavy oil and is used in aluminum smelting and as fuel.
Input power (kWh/kg)
0.1 – 0.3 kWh/kg
(operation of pyrolysis reaction, drying facilities, conveying and cooling systems)
- input fuel
Type: Natural gas, heavy oil
Approximate quantity:
Natural gas: 0.02 – 0.05 kg/kg (used in drying and heating processes)
Heavy oil: 0.01 – 0.03 kg/kg (auxiliary fuel for reactor)
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.05 – 0.1 kg CO₂/kg
(generated by volatile components and residue treatment in the process)
- amount of change in resources such as soil, stone, and water (kg)
Water: 1 – 2 kg/kg (for cooling and cleaning)
- input item name and input amount guideline
Heavy oil (oil residue): 1.1 – 1.3 kg (raw material to obtain 1 kg petroleum coke)
Catalyst (if required): 0.005 – 0.01 kg/kg (used in catalytic cracking)
- process substances such as solvents
Name: Water
Usage: 1 – 2 kg/kg
Circulation rate: 50 – 80% (cooling and cleaning water reuse)
- waste requiring special treatment
Name: Volatile organic compounds (VOCs), wastewater
Approximate quantity: 0.1 – 0.2 kg/kg (exhaust gas treatment required)
Reference Process and Background
Production Process: Heavy oil is pyrolyzed at high temperatures to separate volatile components, producing petroleum coke as a solid carbon residue. The quality of the coke depends on the process temperature and reaction time.
Environmental management: VOC emissions must be controlled and wastewater must be treated appropriately.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.1 – 0.3 kWh/kg |
| Natural gas consumption | 0.02 – 0.05 kg/kg |
| Fuel Oil Consumption | 0.01 – 0.03 kg/kg |
| CO₂ emissions other than combustion | 0.05 – 0.1 kg CO₂/kg |
| Water usage | 1 – 2 kg/kg |
| Heavy oil consumption | 1.1 – 1.3 kg/kg |
| Catalyst usage | 0.005 – 0.01 kg/kg |
| Waste (VOCs, wastewater) | 0.1 – 0.2 kg/kg |
tar pitch
Tar pitch is obtained primarily by distillation or concentration from coal tar and is used in the production of carbon materials and anticorrosion coatings.
Input power (kWh/kg)
0.5 – 1.0 kWh/kg
(used to run the stirrer, distillation column, cooling system, and pumps)
- input fuel
Type: Natural gas, heavy oil
Approximate quantity:
Natural gas: 0.02 – 0.04 kg/kg (used in the heated distillation process)
Heavy oil: 0.01 – 0.03 kg/kg (auxiliary fuel)
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.05 – 0.1 kg CO₂/kg
(by-products of chemical reactions and emissions from flue gas treatment)
- amount of change in resources such as soil, stone, and water (kg)
Water: 1 – 2 kg/kg (used for cooling and cleaning)
- input item name and input amount guideline
Coal tar: 1.2 – 1.5 kg (required to produce 1 kg of tar pitch)
Flocculant (if needed): 0.01 – 0.03 kg
- process substances such as solvents
Name: Water
Usage: 1 – 2 kg/kg
Circulation rate: 60 – 80% (cooling and cleaning water reuse)
- waste requiring special treatment
Name: Distillation residue, wastewater (including organic impurities)
Approximate quantity: 0.2 – 0.4 kg/kg (proper handling required)
Reference Process and Background
Production Process: Coal tar is heated and distilled to obtain pitch. High temperatures are required during distillation, and the waste gases generated in the process must be managed. Flocculants may also be used to adjust the properties of the product.
Environmental management: Organic compounds in distillation residues and wastewater must be treated.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.5 – 1.0 kWh/kg |
| Natural gas consumption | 0.02 – 0.04 kg/kg |
| Fuel Oil Consumption | 0.01 – 0.03 kg/kg |
| CO₂ emissions other than combustion | 0.05 – 0.1 kg CO₂/kg |
| Water usage | 1 – 2 kg/kg |
| Coal tar usage | 1.2 – 1.5 kg/kg |
| Amount of coagulant used | 0.01 – 0.03 kg/kg |
| Waste (residues and wastewater) | 0.2 – 0.4 kg/kg |
Waste tar treatment
Disposal of waste tar is primarily done by pyrolysis, incineration, or chemical treatment and must be controlled to meet environmental standards.
Input power (kWh/kg)
0.8 – 2.0 kWh/kg
(agitation, pumps, temperature control, incinerator or evaporator operation)
- input fuel
Type: Natural gas, heavy oil
Approximate quantity:
Natural gas: 0.03 – 0.06 kg/kg (incinerator or pyrolysis unit heating)
Heavy oil: 0.01 – 0.03 kg/kg (used as auxiliary fuel)
CO₂ emissions other than combustion (kg CO₂/kg)
0.1 – 0.2 kg CO₂/kg
(byproduct of chemical reactions and decomposition of organic compounds)
- amount of change in resources such as soil, stone, and water (kg)
Water: 1 – 2 kg/kg (used for cooling, washing, or evaporation/concentration)
- input item name and input amount guideline
Acid or alkali (neutralizer): 0.05 – 0.1 kg/kg (used to neutralize toxic components)
Flocculant: 0.01 – 0.03 kg/kg (for solids separation)
- process substances such as solvents
Name: Water, solvent (e.g., ethanol, methanol)
Usage: 1 – 2 kg/kg
Circulation rate: 50 – 70% (cooling and cleaning water reuse)
- waste requiring special treatment
Name: Incinerator ash, liquid waste, volatile organic compounds (VOC)
Approximate quantity: 0.2 – 0.5 kg/kg (requires proper treatment and disposal)
Reference Process and Background
Treatment Process: Waste tar is subjected to high-temperature incineration or pyrolysis to remove toxic components. Evaporative concentration and chemical neutralization may also be applied; VOC control and treatment of waste liquids are important.
Environmental management: Waste liquid and incinerated ash must be disposed of and appropriate environmental standards must be met.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.8 – 2.0 kWh/kg |
| Natural gas consumption | 0.03 – 0.06 kg/kg |
| Fuel Oil Consumption | 0.01 – 0.03 kg/kg |
| CO₂ emissions other than combustion | 0.1 – 0.2 kg CO₂/kg |
| Water usage | 1 – 2 kg/kg |
| Amount of neutralizer used | 0.05 – 0.1 kg/kg |
| Amount of coagulant used | 0.01 – 0.03 kg/kg |
| Waste (incinerator ash and liquid waste) | 0.2 – 0.5 kg/kg |
Polymer flocculant (e.g., polyacrylamide, etc.)
Polymer flocculants are widely used in fields such as water treatment and paper manufacturing and are produced through polymerization reactions.
Input power (kWh/kg)
0.5 – 1.2 kWh/kg
(used for agitation, reaction temperature control, drying and filtration equipment, and packaging processes)
- input fuel
Type: Natural gas, electricity (indirect use)
Approximate quantity:
Natural gas: 0.02 – 0.04 kg/kg (used for heating and drying processes)
CO₂ emissions other than combustion (kg CO₂/kg)
0.1 – 0.2 kg CO₂/kg
(emissions of by-products from chemical reactions and solvent use)
- amount of change in resources such as soil, stone, and water (kg)
Water: 2 – 5 kg/kg (for cooling and cleaning)
- input item name and input amount guideline
Acrylic acid or acrylamide: 0.5 – 0.7 kg/kg (main component of polymer)
Initiator (e.g., ammonium persulfate): 0.01 – 0.05 kg/kg (initiates polymerization reaction)
Catalyst or stabilizer: 0.01 – 0.03 kg/kg
- process substances such as solvents
Name: Water, organic solvent (e.g., ethanol, methanol)
Usage: 2 – 5 kg/kg
Circulation rate: 50 – 80% (cooling and cleaning water reuse)
- waste requiring special treatment
Name: Reaction byproducts, unreacted monomers, waste liquids
Approximate quantity: 0.1 – 0.3 kg/kg (proper disposal/recycling required)
Reference Process and Background
Manufacturing Process: Polymer flocculants are produced by polymerization reaction using acrylic acid or acrylamide as the main raw material. Temperature control is critical, and a highly efficient reaction process is required to minimize unreacted monomer.
Environmental management: Recovery and reuse of used solvents and disposal of byproducts are required.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.5 – 1.2 kWh/kg |
| Natural gas consumption | 0.02 – 0.04 kg/kg |
| CO₂ emissions other than combustion | 0.1 – 0.2 kg CO₂/kg |
| Water usage | 2 – 5 kg/kg |
| Amount of acrylic acid or acrylamide used | 0.5 – 0.7 kg/kg |
| Amount of initiator used | 0.01 – 0.05 kg/kg |
| Amount of catalyst or stabilizer used | 0.01 – 0.03 kg/kg |
| Waste (byproducts and effluents) | 0.1 – 0.3 kg/kg |
Dust suppressants
Dust suppressants are manufactured primarily by combining surfactants, binders (adhesives), and polymer solutions to prevent dust dispersion at mining, construction, and transportation sites.
Input power (kWh/kg)
0.3 – 1.0 kWh/kg
(agitation, temperature control, reaction, filtration, drying equipment operation)
- input fuel
Type: Natural gas, electricity (indirect use)
Approximate quantity:
Natural gas: 0.01 – 0.03 kg/kg (used in heating and drying processes)
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.05 – 0.1 kg CO₂/kg
(emissions from chemical reactions and solvent evaporation during production)
- amount of change in resources such as soil, stone, and water (kg)
Water: 1 – 3 kg/kg (for cleaning and cooling)
- input item name and input amount guideline
Surfactant: 0.1 – 0.2 kg/kg (promotes adsorption of dust particles)
Binder (adhesive component): 0.2 – 0.4 kg/kg (for dust agglomeration)
Polymer solution (e.g. polyethylene glycol): 0.3 – 0.5 kg/kg
Preservative: 0.01 – 0.03 kg/kg (extended shelf life)
- process substances such as solvents
Name: Water, organic solvent (if necessary)
Usage: 1 – 3 kg/kg
Circulation rate: 60 – 80% (reuse of cleaning and cooling water)
- waste requiring special treatment
Name: Liquid waste, unreacted materials, volatile organic compounds (VOC)
Approximate quantity: 0.1 – 0.2 kg/kg (proper handling required)
Reference Process and Background
Manufacturing process: Each component is dissolved in water or solvent and mixed uniformly with stirring. Temperature is controlled to ensure proper viscosity and properties. The final product is prepared through drying and filtration processes as necessary.
Environmental Management: Exhaust gas treatment is required to control emissions of volatile organic compounds (VOCs). In addition, waste liquids are either treated appropriately or reused.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.3 – 1.0 kWh/kg |
| Natural gas consumption | 0.01 – 0.03 kg/kg |
| CO₂ emissions other than combustion | 0.05 – 0.1 kg CO₂/kg |
| Water usage | 1 – 3 kg/kg |
| Amount of surfactant used | 0.1 – 0.2 kg/kg |
| Amount of binder used | 0.2 – 0.4 kg/kg |
| Amount of polymer solution used | 0.3 – 0.5 kg/kg |
| Preservative usage | 0.01 – 0.03 kg/kg |
| Waste (liquid waste, VOCs) | 0.1 – 0.2 kg/kg |
Alkali liquid waste treatment
Alkali effluent is mainly controlled by neutralization treatment, which requires appropriate treatment in accordance with the components in the effluent and environmental standards.
Input power (kWh/kg)
0.5 – 1.0 kWh/kg
(agitation, temperature control, filtration equipment, pump operation)
- input fuel
Type: Natural gas, heavy oil
Approximate quantity:
Natural gas: 0.01 – 0.03 kg/kg (if heat treatment is required)
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.01 – 0.05 kg CO₂/kg
(CO₂ byproduct of neutralization reaction)
- amount of change in resources such as soil, stone, and water (kg)
Water: 1 – 2 kg/kg (for cleaning and cooling)
- input item name and input amount guideline
Acid (e.g., sulfuric acid, hydrochloric acid): 0.1 – 0.2 kg/kg (for neutralization)
Flocculant: 0.01 – 0.05 kg/kg (to promote sedimentation)
- process substances such as solvents
Name: Water
Usage: 1 – 2 kg/kg
Circulation rate: 50 – 80% (cooling and cleaning water reuse)
- waste requiring special treatment
Name: Sediment (insoluble salt), effluent
Approximate quantity: 0.1 – 0.3 kg/kg (proper handling required)
Reference Process and Background
Treatment Process: Alkaline effluent is neutralized by acid and, if necessary, a coagulant is added to separate insoluble salts as a precipitate. Finally, the effluent is treated in a manner that meets effluent standards.
Environmental Management: Appropriate treatment of precipitates and effluents generated by the neutralization reaction is required. Landfill or reuse of the precipitates produced by the coagulant will be considered.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.5 – 1.0 kWh/kg |
| Natural gas consumption | 0.01 – 0.03 kg/kg |
| CO₂ emissions other than combustion | 0.01 – 0.05 kg CO₂/kg |
| Water usage | 1 – 2 kg/kg |
| Acid usage | 0.1 – 0.2 kg/kg |
| Amount of coagulant used | 0.01 – 0.05 kg/kg |
| Waste (sediment and liquid waste) | 0.1 – 0.3 kg/kg |
Beneficiation agents (flotation agents, flocculants, etc.)
Beneficiators are chemical mixtures used to improve the efficiency of ore separation and usually contain surfactants, oils, and polymers.
Input power (kWh/kg)
0.3 – 1.0 kWh/kg
(used for agitation, temperature control, reaction equipment, drying and filtration equipment)
- input fuel
Type: Natural gas, heavy oil
Approximate quantity:
Natural gas: 0.01 – 0.03 kg/kg (used in drying and evaporation processes)
CO₂ emissions other than combustion (kg CO₂/kg)
0.05 – 0.1 kg CO₂/kg
(byproduct of chemical reaction and solvent evaporation)
- amount of change in resources such as soil, stone, and water (kg)
Water: 2 – 5 kg/kg (used for cleaning and cooling)
- input item name and input amount guideline
Surfactant: 0.1 – 0.3 kg/kg (for ore surface modification)
Oils (e.g. pine root oil, mineral oil): 0.2 – 0.5 kg/kg (for flotation)
Polymer flocculant: 0.1 – 0.3 kg/kg
Stabilizer (e.g., preservative): 0.01 – 0.03 kg/kg
- process substances such as solvents
Name: Water, organic solvent (e.g., ethanol)
Usage: 2 – 4 kg/kg
Circulation rate: 60 – 80% (cooling water and solvents are reused)
- waste requiring special treatment
Name: Waste liquids, volatile organic compounds (VOCs)
Approximate quantity: 0.1 – 0.3 kg/kg (requires proper treatment and disposal)
Reference Process and Background
Manufacturing Process: Beneficiation agents are made by uniformly mixing and reacting oils, surfactants, and polymers in a solvent. The product is then dried, filtered, and packaged as a product. Temperature control and agitation are required during production, and VOC emission control is required.
Environmental management: Appropriate treatment and reuse of solvents are required to reduce emissions of effluents and VOCs.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.3 – 1.0 kWh/kg |
| Natural gas consumption | 0.01 – 0.03 kg/kg |
| CO₂ emissions other than combustion | 0.05 – 0.1 kg CO₂/kg |
| Water usage | 2 – 5 kg/kg |
| Amount of surfactant used | 0.1 – 0.3 kg/kg |
| Oil usage | 0.2 – 0.5 kg/kg |
| Amount of polymer coagulant used | 0.1 – 0.3 kg/kg |
| Amount of stabilizer used | 0.01 – 0.03 kg/kg |
| Waste (liquid waste, VOCs) | 0.1 – 0.3 kg/kg |
Borax (Borax, Na₂B₄O₇-10H₂O)
Borax is obtained mainly in the dissolution and refining process of borate ores (Colemanite, Cologneite, etc.) and is widely used in the manufacture of detergents and glass.
Input power (kWh/kg)
0.5 – 1.2 kWh/kg
(operation of stirring, dissolution, filtration, and drying equipment)
- input fuel
Type: Natural gas, heavy oil
Approximate quantity:
Natural gas: 0.02 – 0.05 kg/kg (used in heating and drying processes)
CO₂ emissions other than combustion (kg CO₂/kg)
0.05 – 0.1 kg CO₂/kg
(emissions during chemical reactions and refining)
- amount of change in resources such as soil, stone, and water (kg)
Ore removal (tailings): 1.5 – 2.5 kg/kg
Water: 2 – 4 kg/kg (for dissolution and cooling)
- input item name and input amount guideline
Borate ore: 1.5 – 2.0 kg/kg
Acid or alkali (for purification): 0.01 – 0.05 kg/kg (removal of impurities)
- process substances such as solvents
Name: Water
Usage: 2 – 4 kg/kg
Circulation rate: 50 – 70% (cooling and cleaning water reuse)
- waste requiring special treatment
Name: Tailings, wastewater
Approximate quantity: 1.0 – 2.0 kg/kg (requires appropriate treatment or landfill)
Reference Process and Background
Manufacturing process: Borax is obtained by reacting borate ores with water to dissolve them, removing impurities, then filtering and crystallizing the borax. Finally, it is dried to produce a powder or crystalline product.
Environmental management: Tailings management and wastewater treatment are required, and reuse of waste from refining will be considered.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.5 – 1.2 kWh/kg |
| Natural gas consumption | 0.02 – 0.05 kg/kg |
| CO₂ emissions other than combustion | 0.05 – 0.1 kg CO₂/kg |
| Water usage | 2 – 4 kg/kg |
| Amount of borate ore used | 1.5 – 2.0 kg/kg |
| Amount of acid or alkali used | 0.01 – 0.05 kg/kg |
| Waste (tailings, wastewater) | 1.0 – 2.0 kg/kg |
Binder, e.g., cement, binder for carbon products, binder for pellet production, etc.
Binder is widely used to bind and harden powder materials.
Input power (kWh/kg)
0.4 – 1.0 kWh/kg
(mixing, stirring, drying, grinding, and packaging operations)
- input fuel
Type: Natural gas, electricity (indirect use)
Approximate quantity:
Natural gas: 0.02 – 0.05 kg/kg (for heating process and drying)
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.05 – 0.1 kg CO₂/kg
(emissions of by-products from chemical reactions and processes)
- amount of change in resources such as soil, stone, and water (kg)
Water: 1 – 2 kg/kg (used for mixing and cleaning)
- input item name and input amount guideline
Powder materials (e.g. clay, calcium carbonate): 0.6 – 0.8 kg/kg
Polymer or organic material (e.g. starch, polyacrylic acid): 0.1 – 0.3 kg/kg
Additives (e.g., preservatives, curing accelerators): 0.01 – 0.05 kg/kg
- process substances such as solvents
Name: Water, organic solvent (if necessary)
Usage: 1 – 2 kg/kg
Circulation rate: 50 – 80% (reuse of cleaning and cooling water)
- waste requiring special treatment
Name: Liquid waste, dust, unreacted materials
Approximate quantity: 0.1 – 0.2 kg/kg (requires proper disposal or reuse)
Reference Process and Background
Manufacturing process: Powdered and organic materials are mixed and a solvent is added to form a binder. If necessary, the product is dried or pulverized to make the final product. During manufacturing, temperature and humidity control must be strictly monitored to ensure quality.
Environmental management: Dust and liquid waste generated in the manufacturing process must be properly disposed of.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.4 – 1.0 kWh/kg |
| Natural gas consumption | 0.02 – 0.05 kg/kg |
| CO₂ emissions other than combustion | 0.05 – 0.1 kg CO₂/kg |
| Water usage | 1 – 2 kg/kg |
| Amount of powder material used | 0.6 – 0.8 kg/kg |
| Amount of organic materials used | 0.1 – 0.3 kg/kg |
| Amount of additives used | 0.01 – 0.05 kg/kg |
| Waste (liquid waste, dust) | 0.1 – 0.2 kg/kg |
Bisphenol A (BPA)
BPA is mainly produced by the acid-catalyzed condensation reaction of acetone and phenol, and is widely used as a raw material for polycarbonate resin and epoxy resin.
Input power (kWh/kg)
0.4 – 0.8 kWh/kg
(operation of stirrer, reaction control, cooling, filtration and drying processes)
- input fuel
Type: Natural gas, heavy oil
Approximate quantity:
Natural gas: 0.02 – 0.04 kg/kg (heating and drying process)
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.05 – 0.1 kg CO₂/kg
(emissions of chemical reactions and process by-products)
- amount of change in resources such as soil, stone, and water (kg)
Water: 2 – 4 kg/kg (used for cooling and cleaning)
- input item name and input amount guideline
Phenol: 1.5 – 2.0 kg/kg (main raw material)
Acetone: 0.2 – 0.3 kg/kg (for condensation reaction)
Acid catalyst (e.g., hydrochloric or sulfuric acid): 0.01 – 0.03 kg/kg (for reaction acceleration)
- process substances such as solvents
Name: Water, alcohol solvents (if necessary)
Usage: 1 – 2 kg/kg
Recirculation rate: 60 – 80% (cooling and cleaning water reuse)
- waste requiring special treatment
Name: Byproducts (unreacted phenol, pisphenol), effluent
Approximate quantity: 0.2 – 0.3 kg/kg (requires appropriate treatment or reuse)
Reference Process and Background
Production process: Phenol and acetone are condensed under an acid catalyst to produce BPA. After the reaction, the product is cooled and unreacted material is separated. After filtration and drying, BPA is obtained as the final product. Reuse of by-products and unreacted phenol is recommended.
Environmental management: Proper treatment of unreacted materials and liquid waste is required. Reuse of cooling water and cleaning water promotes resource efficiency.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.4 – 0.8 kWh/kg |
| Natural gas consumption | 0.02 – 0.04 kg/kg |
| CO₂ emissions other than combustion | 0.05 – 0.1 kg CO₂/kg |
| Water usage | 2 – 4 kg/kg |
| Phenol usage | 1.5 – 2.0 kg/kg |
| Amount of acetone used | 0.2 – 0.3 kg/kg |
| Acid catalyst usage | 0.01 – 0.03 kg/kg |
| Waste (byproducts and effluents) | 0.2 – 0.3 kg/kg |
Epichlorohydrin (Epichlorohydrin, C₃H₅ClO)
Epichlorohydrin is produced by a process using propylene and chlorine and is widely used as a raw material for epoxy resins.
Input power (kWh/kg)
0.8 – 1.5 kWh/kg
(operation of reactor agitation, cooling system, filtration and distillation facilities)
- input fuel
Type: Natural gas, electricity (indirect use)
Approximate quantity:
Natural gas: 0.03 – 0.06 kg/kg (heating for reaction and distillation processes)
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.05 – 0.1 kg CO₂/kg
(from chemical reactions and by-products)
- amount of change in resources such as soil, stone, and water (kg)
Water: 2 – 5 kg/kg (for cleaning and cooling)
- input item name and input amount guideline
Propylene: 0.8 – 1.0 kg/kg (main raw material)
Chlorine: 0.5 – 0.7 kg/kg (for chlorination process)
Alkali (e.g. sodium hydroxide): 0.1 – 0.2 kg/kg (for neutralization process)
- process substances such as solvents
Name: Water, alcohol solvents (if necessary)
Usage: 1 – 2 kg/kg
Recirculation rate: 60 – 80% (cooling and cleaning water reuse)
- waste requiring special treatment
Name: Byproducts (chlorides, unreacted products), wastewater
Approximate quantity: 0.2 – 0.4 kg/kg (requires proper treatment or reuse)
Reference Process and Background
Production Process: The chlorination process is carried out by reacting propylene with chlorine, followed by a neutralization process to obtain epichlorohydrin. The product is separated by distillation and filtration to become the final product.
Environmental Management: Proper treatment of wastewater containing chlorine and chloride is important. Reuse of cooling water and management of byproducts are also required.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.8 – 1.5 kWh/kg |
| Natural gas consumption | 0.03 – 0.06 kg/kg |
| CO₂ emissions other than combustion | 0.05 – 0.1 kg CO₂/kg |
| Water usage | 2 – 5 kg/kg |
| Propylene usage | 0.8 – 1.0 kg/kg |
| Chlorine usage | 0.5 – 0.7 kg/kg |
| Alkali usage | 0.1 – 0.2 kg/kg |
| Waste (chloride, wastewater) | 0.2 – 0.4 kg/kg |
Ricinoleic Acid
Ricinoleic acid is obtained by hydrolysis and refining processes from castor oil (caster oil) and is used in lubricants, cosmetics, and pharmaceuticals.
Input power (kWh/kg)
0.5 – 1.0 kWh/kg
(for operation of stirring, heating, cooling, and refining processes)
- input fuel
Type: Natural gas, heavy oil
Approximate quantity:
Natural gas: 0.02 – 0.05 kg/kg (heating and distillation process)
CO₂ emissions other than combustion (kg CO₂/kg)
0.05 – 0.1 kg CO₂/kg
(emissions from chemical reactions and by-products during distillation)
- amount of change in resources such as soil, stone, and water (kg)
Water: 2 – 4 kg/kg (for hydrolysis and cooling)
- input item name and input amount guideline
Castor oil: 1.1 – 1.3 kg/kg (main source of ricinoleic acid)
Alkali (e.g. sodium hydroxide): 0.05 – 0.1 kg/kg (for hydrolysis)
Acid (e.g., sulfuric acid): 0.01 – 0.05 kg/kg (for neutralization)
- process substances such as solvents
Name: Water, ethanol (if necessary)
Usage: 1 – 3 kg/kg
Circulation rate: 50 – 70% (reuse of cooling water and cleaning water)
- waste requiring special treatment
Name: Byproduct (glycerin), effluent
Approximate quantity: 0.1 – 0.2 kg/kg (requires proper treatment and reuse)
Reference Process and Background
Production Process: Castor oil is hydrolyzed by alkali treatment to produce ricinoleic acid and glycerin. The ricinoleic acid produced is purified by distillation and filtration. The by-product glycerin is often reused separately.
Environmental management: Waste liquids and by-products generated in the process are properly managed, and reusable resources are recycled.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.5 – 1.0 kWh/kg |
| Natural gas consumption | 0.02 – 0.05 kg/kg |
| CO₂ emissions other than combustion | 0.05 – 0.1 kg CO₂/kg |
| Water usage | 2 – 4 kg/kg |
| Castor oil usage | 1.1 – 1.3 kg/kg |
| Alkali usage | 0.05 – 0.1 kg/kg |
| Acid usage | 0.01 – 0.05 kg/kg |
| Waste (glycerin and liquid waste) | 0.1 – 0.2 kg/kg |
Stabilizers (e.g., for polymers, pharmaceuticals, foods, etc.)
Stabilizers are used in diverse industries to prevent material degradation and involve a variety of raw materials and processes, depending on the type.
Input power (kWh/kg)
0.6 – 1.5 kWh/kg
(used for agitation, reaction temperature control, filtration and drying processes)
- input fuel
Type: Natural gas, heavy oil
Approximate quantity:
Natural gas: 0.01 – 0.04 kg/kg (heating and drying process)
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.05 – 0.1 kg CO₂/kg
(generated as byproduct of chemical reaction)
- amount of change in resources such as soil, stone, and water (kg)
Water: 1 – 3 kg/kg (used for cleaning and cooling)
- input item name and input amount guideline
Organic compounds (e.g., phenols, benzotriazoles): 0.4 – 0.6 kg/kg
Acid or alkali (e.g. sodium hydroxide): 0.1 – 0.2 kg/kg
Additives (e.g., preservatives, antioxidants): 0.01 – 0.05 kg/kg
- process substances such as solvents
Name: Water, organic solvent (e.g., ethanol, methanol)
Usage: 1 – 3 kg/kg
Circulation rate: 60 – 80% (reuse of cooling water and cleaning water)
- waste requiring special treatment
Name: Waste liquids, volatile organic compounds (VOCs), unreacted materials
Approximate quantity: 0.1 – 0.2 kg/kg (requires proper treatment or reuse)
Reference Process and Background
Manufacturing process: Organic compounds are mixed with acids and alkalis and reacted with stirring. The product is then purified, filtered, and dried to make the final product. Temperature and humidity control during the process affects product quality.
Environmental management: Appropriate management of liquid waste and VOCs generated in the manufacturing process is required, and resource efficiency improvement through solvent reuse is recommended.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.6 – 1.5 kWh/kg |
| Natural gas consumption | 0.01 – 0.04 kg/kg |
| CO₂ emissions other than combustion | 0.05 – 0.1 kg CO₂/kg |
| Water usage | 1 – 3 kg/kg |
| Amount of organic compounds used | 0.4 – 0.6 kg/kg |
| Amount of acid or alkali used | 0.1 – 0.2 kg/kg |
| Amount of additives used | 0.01 – 0.05 kg/kg |
| Waste (VOCs, liquid waste) | 0.1 – 0.2 kg/kg |
Stabilizers for polymers
Stabilizers for polymers are used to prevent degradation of plastics and rubbers and are generally manufactured based on hindered phenols or phosphate esters.
Input power (kWh/kg)
0.8 – 1.5 kWh/kg
(stirring, reaction, cooling, filtration, drying process)
- input fuel
Type: Natural gas
Approximate quantity:
Natural gas: 0.02 – 0.04 kg/kg (heating and distillation process)
CO₂ emissions other than combustion (kg CO₂/kg)
0.05 – 0.1 kg CO₂/kg
(from chemical reactions and by-products)
- amount of change in resources such as soil, stone, and water (kg)
Water: 2 – 4 kg/kg (for cooling and cleaning)
- input item name and input amount guideline
Hindered phenol: 0.5 – 0.7 kg/kg (main raw material for antioxidant)
Phosphate: 0.2 – 0.3 kg/kg (degradation inhibitor)
Catalyst (acid or alkali): 0.01 – 0.03 kg/kg (reaction accelerator)
- process substances such as solvents
Name: Water, ethanol
Usage: 1 – 3 kg/kg
Recirculation rate: 60 – 80% (cooling and cleaning water reuse)
- waste requiring special treatment
Name: Unreacted materials, liquid waste, volatile organic compounds (VOC)
Approximate quantity: 0.1 – 0.3 kg/kg (requires appropriate treatment or reuse)
Reference Process and Background
Manufacturing process: Based on hindered phenol or phosphate ester, the product is reacted with the addition of a catalyst, and the product is filtered and dried to make the final product. Cooling water and cleaning water are efficiently reused to reduce environmental impact.
Environmental management: Appropriate treatment of unreacted materials and liquid waste, and control of VOC emissions are required.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.8 – 1.5 kWh/kg |
| Natural gas consumption | 0.02 – 0.04 kg/kg |
| CO₂ emissions other than combustion | 0.05 – 0.1 kg CO₂/kg |
| Water usage | 2 – 4 kg/kg |
| Hindered Phenol Usage | 0.5 – 0.7 kg/kg |
| Amount of phosphate ester used | 0.2 – 0.3 kg/kg |
| Catalyst usage | 0.01 – 0.03 kg/kg |
| Waste (unreacted materials and VOCs) | 0.1 – 0.3 kg/kg |
Polyacrylonitrile (PAN)
PAN is produced through a polymerization reaction using acrylonitrile as the main raw material and is used as a material for carbon fiber, filters, and textile products.
Input power (kWh/kg)
1.0 – 1.5 kWh/kg
(agitation, temperature control, polymerization and drying equipment operation)
- input fuel
Type: Natural gas
Approximate quantity:
Natural gas: 0.02 – 0.05 kg/kg (distillation and drying process)
CO₂ emissions other than combustion (kg CO₂/kg)
0.1 – 0.2 kg CO₂/kg
(from reaction and process by-products)
- amount of change in resources such as soil, stone, and water (kg)
Water: 2 – 4 kg/kg (for cooling and cleaning)
- input item name and input amount guideline
Acrylonitrile: 0.85 – 0.9 kg/kg (main raw material, 85-90% of total reaction)
Methyl acrylate: 0.05 – 0.1 kg/kg (copolymerized component, 5 – 10%)
Initiator (e.g., potassium persulfate): 0.01 – 0.02 kg/kg (for initiating polymerization)
Stabilizer (e.g. polyvinyl alcohol): 0.01 – 0.03 kg/kg (dispersion stabilizer)
- process substances such as solvents
Name: Water, ethanol
Usage: 2 – 4 kg/kg
Circulation rate: 60 – 80% (cooling and cleaning water reuse)
- waste requiring special treatment
Name: Unreacted monomers, liquid waste, volatile organic compounds (VOC)
Approximate quantity: 0.2 – 0.4 kg/kg (requires proper treatment and reuse)
Reference Process and Background
Manufacturing process: Acrylonitrile and methyl acrylate are polymerized in water with an initiator, and the resulting PAN is filtered and dried. The product is used as a precursor for fibers and carbon fibers.
Environmental management: Appropriate treatment of unreacted monomers and liquid waste is required, and the circulation of cooling water reduces environmental impact.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 1.0 – 1.5 kWh/kg |
| Natural gas consumption | 0.02 – 0.05 kg/kg |
| CO₂ emissions other than combustion | 0.1 – 0.2 kg CO₂/kg |
| Water usage | 2 – 4 kg/kg |
| Acrylonitrile usage | 0.85 – 0.9 kg/kg |
| Amount of methyl acrylate used | 0.05 – 0.1 kg/kg |
| Amount of initiator used | 0.01 – 0.02 kg/kg |
| Amount of stabilizer used | 0.01 – 0.03 kg/kg |
| Waste (unreacted monomers and liquid waste) | 0.2 – 0.4 kg/kg |
Oxidation Accelerators for Organic Reactions
Assuming benzoyl peroxide (BPO) as a typical oxidation accelerator, we present data on its production.BPO is widely used in organic chemical reactions as a polymerization initiator and oxidation accelerator.
Input power (kWh/kg)
0.8 – 1.2 kWh/kg
(operation of stirring, cooling, and drying equipment)
- input fuel
Type: Natural gas
Approximate quantity:
Natural gas: 0.02 – 0.04 kg/kg (dry and reaction controlled)
CO₂ emissions other than combustion (kg CO₂/kg)
0.1 – 0.2 kg CO₂/kg
(emissions from side reactions and process origin)
- amount of change in resources such as soil, stone, and water (kg)
Water: 2 – 3 kg/kg (used for cooling and cleaning)
- input item name and input amount guideline
Benzoyl chloride: 0.6 – 0.7 kg/kg (main raw material for oxidation accelerator)
Hydrogen peroxide: 0.3 – 0.4 kg/kg (oxidizer)
Calcium chloride: 0.05 – 0.1 kg/kg (drying accelerator)
- process substances such as solvents
Name: Water, ethyl acetate
Usage: 1 – 2 kg/kg
Circulation rate: 60 – 75% (reuse of cooling and cleaning water)
- waste requiring special treatment
Name: Unreacted materials, liquid waste, volatile organic compounds (VOC)
Approximate quantity: 0.1 – 0.3 kg/kg (proper handling required)
Reference Process and Background
Manufacturing process: Benzoyl chloride reacts with hydrogen peroxide to produce benzoyl peroxide. The product is filtered and dried to finish as a final product. Reuse of by-products and unreacted materials is recommended.
Environmental management: VOC emissions and appropriate treatment of unreacted materials are required. Emphasis will be placed on reducing environmental impact through recycling of solvents.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.8 – 1.2 kWh/kg |
| Natural gas consumption | 0.02 – 0.04 kg/kg |
| CO₂ emissions other than combustion | 0.1 – 0.2 kg CO₂/kg |
| Water usage | 2 – 3 kg/kg |
| Benzoyl chloride usage | 0.6 – 0.7 kg/kg |
| Amount of hydrogen peroxide used | 0.3 – 0.4 kg/kg |
| Calcium chloride usage | 0.05 – 0.1 kg/kg |
| Waste (unreacted materials and VOCs) | 0.1 – 0.3 kg/kg |
Desulfurization catalysts (typically molybdenum-based catalysts, Co-Mo/Al₂O₃ catalysts, etc.)
These catalysts are used in oil refining and natural gas processing to remove sulfur compounds.
Input power (kWh/kg)
1.0 – 1.8 kWh/kg
(mixing, dissolving, stirring, drying, and firing equipment operation)
- input fuel
Type: Natural gas
Approximate quantity:
Natural gas: 0.03 – 0.06 kg/kg (for heating the firing process)
CO₂ emissions other than combustion (kg CO₂/kg)
0.1 – 0.2 kg CO₂/kg
(emissions from chemical reaction by-products and process gas treatment)
- amount of change in resources such as soil, stone, and water (kg)
Water: 3 – 5 kg/kg (used for cleaning and cooling)
- input item name and input amount guideline
Aluminum oxide (Al₂O₃): 0.5 – 0.6 kg/kg (used as carrier)
Molybdenum oxide (MoO₃): 0.2 – 0.3 kg/kg (active ingredient)
Cobalt nitrate: 0.1 – 0.15 kg/kg (used as an auxiliary catalyst)
Barium oxide (BaO): 0.01 – 0.03 kg/kg (accelerant)
- process substances such as solvents
Name: Water, Nitric acid
Usage: 3 – 5 kg/kg
Circulation rate: 50 – 70% (reuse of cleaning and cooling water)
- waste requiring special treatment
Name: Waste liquid (containing nitrate), VOC in exhaust gas
Approximate quantity: 0.3 – 0.5 kg/kg (proper handling required)
Reference Process and Background
Manufacturing process: Aluminum oxide is used as a support, molybdenum and cobalt nitrates are dissolved, and the solution is impregnated into the support, which is then dried and calcined. This immobilizes the active components and produces a high-performance desulfurization catalyst.
Environmental management: Proper treatment of liquid waste containing nitric acid is required; exhaust gas treatment to reduce VOC emissions is important.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 1.0 – 1.8 kWh/kg |
| Natural gas consumption | 0.03 – 0.06 kg/kg |
| CO₂ emissions other than combustion | 0.1 – 0.2 kg CO₂/kg |
| Water usage | 3 – 5 kg/kg |
| Amount of aluminum oxide used | 0.5 – 0.6 kg/kg |
| Amount of molybdenum oxide used | 0.2 – 0.3 kg/kg |
| Amount of cobalt nitrate used | 0.1 – 0.15 kg/kg |
| Amount of barium oxide used | 0.01 – 0.03 kg/kg |
| Waste (liquid waste, VOCs) | 0.3 – 0.5 kg/kg |
Ethylene glycol (Ethylene Glycol, C₂H₆O₂)
Ethylene glycol is obtained primarily through the process of ethylene oxidation and hydrolysis and is used as a raw material for automotive antifreeze solutions and polyester resins.
Input power (kWh/kg)
0.5 – 1.2 kWh/kg
(used for reaction, temperature control, separation and purification processes)
- input fuel
Type: Natural gas
Approximate quantity:
Natural gas: 0.02 – 0.04 kg/kg (heating and distillation process)
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.1 – 0.15 kg CO₂/kg
(by-products of the reaction and emissions from flue gas treatment)
- amount of change in resources such as soil, stone, and water (kg)
Water: 3 – 5 kg/kg (used for reaction and cooling)
- input item name and input amount guideline
Ethylene: 0.6 – 0.7 kg/kg (main raw material for oxidation reaction)
Oxygen: 0.3 – 0.4 kg/kg (used for ethylene oxidation)
Water: 1.5 – 2.5 kg/kg (used for hydrolysis)
- process substances such as solvents
Name: Water, methanol (if necessary)
Usage: 2 – 4 kg/kg
Recirculation rate: 70 – 85% (cooling and cleaning water reuse)
- waste requiring special treatment
Name: Unreacted ethylene, wastewater (containing organic matter)
Approximate quantity: 0.2 – 0.4 kg/kg (proper handling required)
Reference Process and Background
Production process: Ethylene oxide is produced by the reaction of ethylene and oxygen, followed by reaction with water to obtain ethylene glycol. Small amounts of diethylene glycol and triethylene glycol are produced as byproducts.
Environmental management: Reuse of unreacted ethylene and wastewater treatment are required. Reuse of cooling water from the distillation process is also common.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.5 – 1.2 kWh/kg |
| Natural gas consumption | 0.02 – 0.04 kg/kg |
| CO₂ emissions other than combustion | 0.1 – 0.15 kg CO₂/kg |
| Water usage | 3 – 5 kg/kg |
| Ethylene usage | 0.6 – 0.7 kg/kg |
| Oxygen usage | 0.3 – 0.4 kg/kg |
| Water input (for hydrolysis) | 1.5 – 2.5 kg/kg |
| Waste (unreacted materials and wastewater) | 0.2 – 0.4 kg/kg |
Polymerization accelerating catalyst (typical examples: dialkylaluminum compounds, Ziegler-Natta catalysts)
They are widely used in olefin polymerization, including the production of polypropylene and polyethylene.
Input power (kWh/kg)
1.0 – 2.0 kWh/kg
(reaction temperature control, agitation, filtration, drying facility operation)
- input fuel
Type: Natural gas
Approximate quantity:
Natural gas: 0.03 – 0.05 kg/kg (drying and distillation process)
CO₂ emissions other than combustion (kg CO₂/kg)
0.1 – 0.15 kg CO₂/kg
(emissions from chemical reactions and by-products)
- amount of change in resources such as soil, stone, and water (kg)
Water: 3 – 6 kg/kg (for cooling and cleaning)
- input item name and input amount guideline
Titanium trichloride (TiCl₃): 0.4 – 0.6 kg/kg (active core component)
Diethylaluminum Chloride (DEAC): 0.3 – 0.4 kg/kg (auxiliary catalyst)
Magnesium chloride (MgCl₂): 0.2 – 0.3 kg/kg (support stabilization)
Organophosphorus compounds: 0.01 – 0.02 kg/kg (reaction control agent)
- process substances such as solvents
Name: Hexane, Toluene
Usage: 1 – 2 kg/kg
Recirculation rate: 70 – 85% (reused for cooling and cleaning)
- waste requiring special treatment
Name: Unreacted materials, liquid waste, VOCs
Approximate quantity: 0.2 – 0.4 kg/kg (proper handling required)
Reference Process and Background
Production Process: Titanium trichloride is reacted with diethylaluminum chloride to produce a polymerization catalyst. The product is stabilized with magnesium chloride, then dried and filtered to yield the product. Reuse of by-products and unreacted materials is recommended.
Environmental management: Reuse of solvents and disposal of unreacted materials are important. Reduction of effluent and VOC emissions is also necessary.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 1.0 – 2.0 kWh/kg |
| Natural gas consumption | 0.03 – 0.05 kg/kg |
| CO₂ emissions other than combustion | 0.1 – 0.15 kg CO₂/kg |
| Water usage | 3 – 6 kg/kg |
| Amount of titanium trichloride used | 0.4 – 0.6 kg/kg |
| Diethylaluminum Chloride Usage | 0.3 – 0.4 kg/kg |
| Amount of magnesium chloride used | 0.2 – 0.3 kg/kg |
| Amount of organophosphorus compounds used | 0.01 – 0.02 kg/kg |
| Waste (unreacted materials and VOCs) | 0.2 – 0.4 kg/kg |
Paraben
A typical paraben is methylparaben (Methylparaben), which is widely used in cosmetics and pharmaceuticals as a preservative.
Input power (kWh/kg)
0.5 – 1.0 kWh/kg
(reaction temperature control, stirring, purification and filtration processes)
- input fuel
Type: Natural gas
Approximate quantity:
Natural gas: 0.02 – 0.03 kg/kg (drying and distillation process)
CO₂ emissions other than combustion (kg CO₂/kg)
0.05 – 0.1 kg CO₂/kg
(by-products of the reaction and distillation process)
- amount of change in resources such as soil, stone, and water (kg)
Water: 2 – 3 kg/kg (for cleaning and cooling)
- input item name and input amount guideline
p-Hydroxybenzoic acid: 0.7 – 0.8 kg/kg (main raw material)
Methanol: 0.2 – 0.3 kg/kg (alcohol for esterification)
Sulfuric acid: 0.01 – 0.02 kg/kg (acid catalyst)
- process substances such as solvents
Name: Water, Methanol
Usage: 2 – 4 kg/kg
Circulation rate: 70 – 80% (cooling and cleaning water reuse)
- waste requiring special treatment
Name: Unreacted materials, liquid waste, methanol vapor
Approximate quantity: 0.1 – 0.2 kg/kg (requires proper exhaust and treatment)
Reference Process and Background
Production process: p-hydroxybenzoic acid and methanol are reacted with sulfuric acid as a catalyst to form an ester. The reaction product is cooled, filtered, and dried to yield the final product, methylparaben.
Environmental management: Appropriate treatment of methanol vapor, a byproduct, is required. Environmental impact is reduced through the use of circulating cooling water and cleaning water.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.5 – 1.0 kWh/kg |
| Natural gas consumption | 0.02 – 0.03 kg/kg |
| CO₂ emissions other than combustion | 0.05 – 0.1 kg CO₂/kg |
| Water usage | 2 – 3 kg/kg |
| Amount of p-hydroxybenzoic acid used | 0.7 – 0.8 kg/kg |
| Methanol usage | 0.2 – 0.3 kg/kg |
| Sulfuric Acid Usage | 0.01 – 0.02 kg/kg |
| Waste (unreacted materials and liquid waste) | 0.1 – 0.2 kg/kg |
Acetic Acid
As a typical production process, the carbonylation method (Monsanto method, Cativa method) using methanol as feedstock is often used.
Input power (kWh/kg)
0.4 – 0.8 kWh/kg
(stirring, temperature control, distillation and filtration equipment operation)
- input fuel
Type: Natural gas
Approximate quantity:
Natural gas: 0.02 – 0.05 kg/kg (heating and distillation process)
CO₂ emissions other than combustion (kg CO₂/kg)
0.05 – 0.1 kg CO₂/kg
(byproducts from the reaction and process)
- amount of change in resources such as soil, stone, and water (kg)
Water: 2 – 4 kg/kg (for reaction, cooling and washing)
- input item name and input amount guideline
Methanol: 0.3 – 0.4 kg/kg (for carbonylation reaction)
Carbon monoxide: 0.5 – 0.6 kg/kg (for carbonylation reaction)
Ruthenium iodide: 0.005 – 0.01 kg/kg (catalyst)
- process substances such as solvents
Name: Water, Methanol
Usage: 1 – 3 kg/kg
Circulation rate: 70 – 85% (reuse of cooling and cleaning water)
- waste requiring special treatment
Name: Unreacted methanol, wastewater (containing organic matter)
Approximate quantity: 0.1 – 0.2 kg/kg (proper handling required)
Reference Process and Background
Production Process: Methanol and carbon monoxide react under a catalyst to produce acetic acid. Catalysts such as ruthenium iodide are used in this reaction, and distillation and filtration are required to purify the product.
Environmental management: Reuse of unreacted methanol and treatment of wastewater are required. Reuse of cooling water reduces environmental impact.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.4 – 0.8 kWh/kg |
| Natural gas consumption | 0.02 – 0.05 kg/kg |
| CO₂ emissions other than combustion | 0.05 – 0.1 kg CO₂/kg |
| Water usage | 2 – 4 kg/kg |
| Methanol usage | 0.3 – 0.4 kg/kg |
| Carbon monoxide usage | 0.5 – 0.6 kg/kg |
| Amount of ruthenium iodide used | 0.005 – 0.01 kg/kg |
Palladium (Pd)
Palladium is obtained mainly by extraction and refining from PGM ores (platinum group metal ores) and is widely used in catalysts and electronic components.
Input power (kWh/kg)
20 – 30 kWh/kg
(used for ore crushing, flotation, dissolution, electrolytic refining, and filtration)
- input fuel
Type: Heavy oil, natural gas
Approximate quantity:
Heavy oil: 0.5 – 1.0 kg/kg (for processing in high-temperature furnaces)
Natural gas: 0.1 – 0.2 kg/kg (for drying and refining processes)
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.5 – 1.0 kg CO₂/kg
(byproduct of acid treatment and electrolysis)
- amount of change in resources such as soil, stone, and water (kg)
Ore throughput: 300 – 500 kg/kg (extracted from large amounts of ore)
Water: 50 – 100 kg/kg (for cooling and cleaning)
- input item name and input amount guideline
PGM ore: 300 – 500 kg/kg (main raw material)
Nitric acid: 10 – 20 kg/kg (dissolution and impurity removal)
Hydrochloric acid: 5 – 10 kg/kg (purification and solution preparation)
- process substances such as solvents
Name: Water, hydrochloric acid
Usage: 50 – 100 kg/kg
Circulation rate: 60 – 80% (cooling water reuse)
- waste requiring special treatment
Name: Metal components in tailings, acid waste, and flue gas
Approximate quantity: 30 – 50 kg/kg (requires proper treatment of effluent and tailings)
Reference Process and Background
Production process: Palladium is extracted from ores by flotation or dissolution and refined by electrolysis. Removal of impurities by the acid used is important, and large amounts of ore must be processed. Proper treatment of by-products and unused acid is required.
Environmental management: Neutralization of acid waste liquid and tailings management are required. Environmental impact is reduced through reuse of cooling water and exhaust gas treatment.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 20 – 30 kWh/kg |
| Fuel Oil Consumption | 0.5 – 1.0 kg/kg |
| Natural gas consumption | 0.1 – 0.2 kg/kg |
| CO₂ emissions other than combustion | 0.5 – 1.0 kg CO₂/kg |
| Ore processing volume | 300 – 500 kg/kg |
| Water usage | 50 – 100 kg/kg |
| Nitric acid usage | 10 – 20 kg/kg |
| Hydrochloric acid usage | 5 – 10 kg/kg |
| Waste (tailings, acid waste) | 30 – 50 kg/kg |
Fluorospar (Fluorspar, CaF₂)
Fluorospar is mainly used in the production of hydrofluoric acid and aluminum refining, and is industrially produced through mining and flotation from ore.
Input power (kWh/kg)
0.4 – 0.7 kWh/kg
(ore crushing, flotation, drying, sorting)
- input fuel
Type: Light oil
Approximate quantity:
Diesel oil: 0.02 – 0.05 kg/kg (mining and transportation process)
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.01 – 0.05 kg CO₂/kg
(emissions from grinding and flotation processes)
- amount of change in resources such as soil, stone, and water (kg)
Ore throughput: 5 – 10 kg/kg (including waste tailings)
Water: 3 – 6 kg/kg (used for washing and flotation)
- input item name and input amount guideline
Fluorite ore (CaF₂ ore): 5 – 10 kg/kg (main raw material)
Flotation agent (e.g., xylene compounds): 0.01 – 0.03 kg/kg (for flotation separation of ores)
Flocculant (e.g. polymer-based flocculant): 0.005 – 0.01 kg/kg (used in flotation process)
- process substances such as solvents
Name: Water
Usage: 3 – 6 kg/kg
Circulation rate: 60 – 75% (cooling and cleaning water reuse)
- waste requiring special treatment
Name: Waste tailings, flotation waste
Approximate quantity: 4 – 8 kg/kg (requires proper handling)
Reference Process and Background
Manufacturing process: Fluorite ore is mined, crushed, and flotation is performed to remove impurities. The product is then dried and sorted to obtain high-purity fluorospar. Large amounts of tailings are generated as by-products, and water reuse is recommended.
Environmental management: Management of tailings and effluents is important, as is wastewater treatment of chemicals used in flotation.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.4 – 0.7 kWh/kg |
| Diesel oil consumption | 0.02 – 0.05 kg/kg |
| CO₂ emissions other than combustion | 0.01 – 0.05 kg CO₂/kg |
| Ore processing volume | 5 – 10 kg/kg |
| Water usage | 3 – 6 kg/kg |
| Flotation agent usage | 0.01 – 0.03 kg/kg |
| Amount of coagulant used | 0.005 – 0.01 kg/kg |
| Waste (tailings, liquid waste) | 4 – 8 kg/kg |
Potassium hydroxide (KOH)
The electrolytic method (electrolysis of an aqueous solution of potassium chloride) is commonly used as the main production method.
Input power (kWh/kg)
2.0 – 2.5 kWh/kg
(electrolysis process, stirring, filtration and drying process)
- input fuel
Type: Natural gas
Approximate quantity:
Natural gas: 0.05 – 0.1 kg/kg (heating and drying process)
CO₂ emissions other than combustion (kg CO₂/kg)
0.1 – 0.2 kg CO₂/kg
(byproducts from the reaction process)
- amount of change in resources such as soil, stone, and water (kg)
Water: 3 – 5 kg/kg (for dissolution, washing and cooling)
- input item name and input amount guideline
Potassium chloride (KCl): 1.6 – 1.8 kg/kg (electrolytic raw material)
Water: 2.5 – 3.5 kg/kg (for dissolution)
- process substances such as solvents
Name: Water
Usage: 3 – 5 kg/kg
Recirculation rate: 80 – 90% (cooling and cleaning water reuse)
- waste requiring special treatment
Name: Waste liquid (including residual chloride)
Approximate quantity: 0.2 – 0.3 kg/kg (requires appropriate wastewater treatment)
Reference Process and Background
Manufacturing Process: Potassium chloride solution is electrolyzed to produce potassium hydroxide, chlorine, and hydrogen. The KOH produced is concentrated and dried and shipped as a solid or concentrated solution. The chlorine and hydrogen produced by electrolysis can be recovered for other uses.
Environmental Management: Unused chlorides and effluents must be disposed of. Reuse of by-products (hydrogen and chlorine) generated in the process is also encouraged.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 2.0 – 2.5 kWh/kg |
| Natural gas consumption | 0.05 – 0.1 kg/kg |
| CO₂ emissions other than combustion | 0.1 – 0.2 kg CO₂/kg |
| Water usage | 3 – 5 kg/kg |
| Amount of potassium chloride used | 1.6 – 1.8 kg/kg |
| Waste liquid discharged | 0.2 – 0.3 kg/kg |
Calcium hydroxide (Ca(OH)₂)
Typical production methods include calcination of limestone and hydration reactions, and it is widely used in construction materials and the chemical industry.
Input power (kWh/kg)
0.2 – 0.5 kWh/kg
(used for grinding, stirring, filtration and drying processes)
- input fuel
Type: Natural gas, coke
Approximate quantity:
Natural gas: 0.02 – 0.04 kg/kg
Coke: 0.1 – 0.15 kg/kg
(used in limestone calcination process)
CO₂ emissions other than combustion (kg CO₂/kg)
0.7 – 0.9 kg CO₂/kg
(CO₂ release from thermal decomposition of limestone (CaCO₃))
- amount of change in resources such as soil, stone, and water (kg)
Amount of limestone processed: 1.8 – 2.0 kg/kg (CaCO₃ → CaO → Ca(OH)₂)
Water: 0.4 – 0.6 kg/kg (hydration process)
- input item name and input amount guideline
Limestone (CaCO₃): 1.8 – 2.0 kg/kg (main raw material)
Water: 0.4 – 0.6 kg/kg (for hydration reaction)
- process substances such as solvents
Name: Water
Usage: 0.4 – 0.6 kg/kg
Circulation rate: 50 – 70% (reuse of cooling and cleaning water)
- waste requiring special treatment
Name: Unreacted materials, liquid waste (sludge containing impurities)
Approximate quantity: 0.1 – 0.2 kg/kg (proper handling required)
Reference Process and Background
Manufacturing Process: First, limestone (CaCO₃) is calcined to obtain quicklime (CaO). Then, calcium hydroxide (Ca(OH)₂) is formed by reaction with water. Because the calcination process generates a large amount of CO₂, environmental measures are important.
Environmental Management: Reduction and recovery of CO₂ emissions during lime calcination is required. Reuse of cooling water and proper treatment of sludge contribute to the reduction of environmental impact.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.2 – 0.5 kWh/kg |
| Natural gas consumption | 0.02 – 0.04 kg/kg |
| Coke consumption | 0.1 – 0.15 kg/kg |
| CO₂ emissions other than combustion | 0.7 – 0.9 kg CO₂/kg |
| Amount of limestone used | 1.8 – 2.0 kg/kg |
| Water usage | 0.4 – 0.6 kg/kg |
| Waste (unreacted materials and sludge) | 0.1 – 0.2 kg/kg |
Heavy fuel oil (HFO)
Heavy crude oil is produced from the heavier fractions obtained during the crude oil refining process and is used in marine fuels and industrial boilers. Typical processes include atmospheric and vacuum distillation and contact reforming.
Input power (kWh/kg)
0.05 – 0.1 kWh/kg
(used for pump, agitation, temperature control)
- input fuel
Type: Natural gas, light oil
Approximate quantity:
Natural gas: 0.01 – 0.03 kg/kg (heating process)
Diesel oil: 0.02 – 0.04 kg/kg (process operating aid)
CO₂ emissions other than combustion (kg CO₂/kg)
0.05 – 0.1 kg CO₂/kg
(byproduct of desulfurization and reforming processes)
- amount of change in resources such as soil, stone, and water (kg)
Water: 1.5 – 2.0 kg/kg (for cleaning and cooling)
Crude oil throughput: 2.0 – 2.5 kg/kg (crude oil processing to obtain heavy oil)
- input item name and input amount guideline
Crude oil: 2.0 – 2.5 kg/kg (main raw material)
Hydrogen: 0.01 – 0.02 kg/kg (used for desulfurization and reforming)
Catalyst (alumina-based): 0.005 – 0.01 kg/kg (for desulfurization reaction)
- process substances such as solvents
Name: Water
Usage: 1.5 – 2.0 kg/kg
Circulation rate: 70 – 85% (cooling and cleaning water reuse)
- waste requiring special treatment
Name: Waste catalyst, wastewater, heavy oil sludge
Approximate quantity: 0.1 – 0.3 kg/kg (proper handling required)
Reference Process and Background
Production process: Crude oil is separated by atmospheric distillation and vacuum distillation, and the resulting heavy fraction is desulfurized using hydrogen to produce heavy oil. Since catalysts are used, proper treatment of waste catalysts is required. Reuse of cooling water and management of byproducts contribute to the reduction of environmental impact.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.05 – 0.1 kWh/kg |
| Natural gas consumption | 0.01 – 0.03 kg/kg |
| Diesel oil consumption | 0.02 – 0.04 kg/kg |
| CO₂ emissions other than combustion | 0.05 – 0.1 kg CO₂/kg |
| Crude oil usage | 2.0 – 2.5 kg/kg |
| Hydrogen usage | 0.01 – 0.02 kg/kg |
| Catalyst usage | 0.005 – 0.01 kg/kg |
| Water usage | 1.5 – 2.0 kg/kg |
| Waste (waste catalyst and wastewater) | 0.1 – 0.3 kg/kg |
Catalysts for catalytic cracking (typical example: fluidized catalytic cracking (FCC) catalysts)
FCC catalysts are used to crack heavy petroleum fractions and are typically alumina- or zeolite-based materials.
Input power (kWh/kg)
2.0 – 3.5 kWh/kg
(stirring, firing, drying, filtering and mixing processes)
- input fuel
Type: Natural gas
Approximate quantity:
Natural gas: 0.05 – 0.1 kg/kg (firing and drying process)
CO₂ emissions other than combustion (kg CO₂/kg)
0.1 – 0.2 kg CO₂/kg
(emissions in the firing process)
- amount of change in resources such as soil, stone, and water (kg)
Water: 3 – 5 kg/kg (for cooling and cleaning)
- input item name and input amount guideline
Alumina (Al₂O₃): 0.5 – 0.7 kg/kg (carrier component)
Zeolite: 0.2 – 0.3 kg/kg (active material)
Silica (SiO₂): 0.1 – 0.2 kg/kg (auxiliary component)
- process substances such as solvents
Name: Water, sulfuric acid
Usage: 3 – 4 kg/kg
Recirculation rate: 60 – 75% (reuse of cleaning and cooling water)
- waste requiring special treatment
Name: Trace metal components in unused alumina, liquid waste, and exhaust gas
Approximate volume: 0.2 – 0.4 kg/kg (requires proper exhaust and effluent treatment)
Reference Process and Background
Manufacturing process: Alumina and zeolite are mixed, stirred, and calcined at high temperature. After calcination, the catalyst is dried and shaped. Water and sulfuric acid are used to prepare the catalyst, and unused materials and waste liquids must be reused.
Environmental management: CO₂ emissions from the firing process and unused materials in wastewater need to be properly treated. Reuse of cooling water and solvents improves resource efficiency.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 2.0 – 3.5 kWh/kg |
| Natural gas consumption | 0.05 – 0.1 kg/kg |
| CO₂ emissions other than combustion | 0.1 – 0.2 kg CO₂/kg |
| Water usage | 3 – 5 kg/kg |
| Alumina usage | 0.5 – 0.7 kg/kg |
| Zeolite usage | 0.2 – 0.3 kg/kg |
| Silica usage | 0.1 – 0.2 kg/kg |
| Waste (unused materials and waste liquids) | 0.2 – 0.4 kg/kg |
Volatile Organic Compounds (VOC) Treatment
VOCs are typically treated by thermal oxidation (TO: Thermal Oxidation) or catalytic oxidation (Catalytic Oxidation).
Input power (kWh/kg)
0.3 – 0.8 kWh/kg
(used for agitation, operation of gas treatment systems, air supply)
- input fuel
Type: Natural gas
Approximate quantity:
Natural gas: 0.1 – 0.15 kg/kg (for combustion, maintaining oxidation temperature)
CO₂ emissions other than combustion (kg CO₂/kg)
0.1 – 0.2 kg CO₂/kg
(byproduct from chemical reaction)
- amount of change in resources such as soil, stone, and water (kg)
Water: 1.0 – 2.0 kg/kg (for cooling and cleaning)
- input item name and input amount guideline
Natural gas: 0.1 – 0.15 kg/kg (energy supply for oxidation reactions)
Oxygen (air component): 10 – 12 kg/kg (source of oxidation reaction)
Catalyst (precious metal based, e.g. palladium catalyst): 0.005 – 0.01 kg/kg (for catalytic oxidation method)
- process substances such as solvents
Name: Water
Usage: 1.0 – 2.0 kg/kg
Recirculation rate: 80 – 90% (cooling and cleaning water reuse)
- waste requiring special treatment
Name: Residue in exhaust gas, waste catalyst
Approximate quantity: 0.1 – 0.2 kg/kg (waste catalyst must be reused or disposed of properly)
Reference Process and Background
In the thermal oxidation method, VOCs are combusted at 850°C or higher to decompose them into carbon dioxide and water. In the catalytic oxidation method, a catalyst is used to accelerate the oxidation reaction at a low temperature of 200 to 300℃. This method efficiently processes VOCs while reducing fuel consumption.
Environmental management: Trace constituents in exhaust gas after VOC treatment must be managed and waste catalyst must be treated appropriately.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.3 – 0.8 kWh/kg |
| Natural gas consumption | 0.1 – 0.15 kg/kg |
| CO₂ emissions other than combustion | 0.1 – 0.2 kg CO₂/kg |
| Water usage | 1.0 – 2.0 kg/kg |
| Oxygen (airborne component) usage | 10 – 12 kg/kg |
| Catalyst (precious metal-based) | 0.005 – 0.01 kg/kg |
| Waste (waste catalyst and exhaust gas residue) | 0.1 – 0.2 kg/kg |
coal tar
Coal tar is a byproduct of the process of high-temperature dry distillation (heating in coke ovens, etc.) of coal and is used as a raw material in the chemical industry.
Input power (kWh/kg)
0.1 – 0.3 kWh/kg
(conveying, pumps, temperature control system in dry distillation process)
- input fuel
Type: Coke gas, natural gas
Approximate quantity:
Coke gas: 0.05 – 0.1 kg/kg
Natural gas: 0.01 – 0.03 kg/kg (heating and drying aid)
CO₂ emissions other than combustion (kg CO₂/kg)
0.3 – 0.5 kg CO₂/kg
(emissions from gas released during dry distillation process)
- amount of change in resources such as soil, stone, and water (kg)
Water: 1 – 2 kg/kg (used for cooling and cleaning)
Coal usage: 5 – 6 kg/kg (raw material for dry distillation)
- input item name and input amount guideline
Coal: 5 – 6 kg/kg (main raw material)
Coke gas: 0.05 – 0.1 kg/kg (heating energy)
Cooling water: 1 – 2 kg/kg (for cleaning and cooling)
- process substances such as solvents
Name: Water
Usage: 1 – 2 kg/kg
Recirculation rate: 70 – 85% (cooling and cleaning water reuse)
- waste requiring special treatment
Name: Tar sludge, organic components in wastewater
Approximate quantity: 0.2 – 0.4 kg/kg (proper handling required)
Reference Process and Background
Production Process: Coal is dry distilled at high temperatures to produce gas, coke, and tar. The dry distillation temperature is 900-1000°C. The tar is separated along with the gas. Cooling and rinse water circulation is recommended, and treatment of sludge and wastewater as by-products is required.
Environmental management: Proper treatment of organic components and tar sludge in wastewater is important. Reuse of cooling water reduces environmental impact.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.1 – 0.3 kWh/kg |
| Coke gas consumption | 0.05 – 0.1 kg/kg |
| Natural gas consumption | 0.01 – 0.03 kg/kg |
| CO₂ emissions other than combustion | 0.3 – 0.5 kg CO₂/kg |
| Coal usage | 5 – 6 kg/kg |
| Water usage | 1 – 2 kg/kg |
| Waste (tar sludge, wastewater) | 0.2 – 0.4 kg/kg |
neutralizer
The following is a guide to typical neutralizers and the percentage (share) of neutralizers used. The choice of neutralizer depends on the nature of the acid or alkali to be treated, cost, and speed of reaction. The figures below are approximate market shares for typical industrial applications (wastewater treatment, chemical manufacturing, etc.).
Typical neutralizers and their market share
| neutralizer | use | Share in use (%) |
| Sodium hydroxide (NaOH) | Neutralization of acidic wastewater and industrial effluent | 40 – 50%. |
| Lime (calcium hydroxide, Ca(OH)₂) | Wastewater treatment, flue gas desulfurization | 25 – 30 |
| Sodium carbonate (Na₂CO₃) | Neutralization of mildly acidic liquid waste | 10 – 15% of |
| Ammonia (NH₃) | Wastewater treatment, chemical process | 5 – 10% |
| Sodium bicarbonate (NaHCO₃) | Chemical manufacturing, food industry | 5 – 10% |
Selection Criteria and Applications of Neutralizers
Sodium hydroxide (NaOH)
It has strong alkalinity and is used to regulate acidic wastewater and chemical reactions.
The size of the market share is due to its low cost and immediate effect.
Lime (calcium hydroxide, Ca(OH)₂)
Used in wastewater treatment, flue gas desulfurization, etc., it is low cost and suitable for mass processing.
The challenge is that the sludge generated needs to be treated.
Sodium carbonate (Na₂CO₃)
It is used to neutralize mildly acidic solutions.
Reaction is milder and safer than sodium hydroxide.
Ammonia (NH₃)
Used in wastewater treatment and chemical processes for more flexible pH control.
Can be volatile and difficult to manage.
Sodium bicarbonate (NaHCO₃)
As a mild neutralizer, it is used in food and pharmaceutical manufacturing processes.
It is used in situations where pH adjustment is highly accurate and safety is important.
summary
The share of neutralizers used depends primarily on cost, immediacy, safety, and handling of byproducts. A summary of market shares is reproduced below:
Sodium hydroxide (NaOH): 40 – 50%.
Lime (calcium hydroxide): 25 – 30%.
Sodium carbonate (Na₂CO₃): 10 – 15
Ammonia (NH₃): 5 – 10%
Sodium bicarbonate (NaHCO₃): 5 – 10%
Thus, the choice of neutralizer is optimized according to the application.
surfactant
(Typical examples: sodium alkyl benzene sulfonate, alcohol ethoxylates, etc.)
Surfactants are widely used as emulsifiers in detergents, cosmetics, and industrial applications.
Input power (kWh/kg)
0.5 – 1.2 kWh/kg
(agitation, temperature control, pumps, filtration and drying processes)
- input fuel
Type: Natural gas
Approximate quantity:
Natural gas: 0.02 – 0.05 kg/kg (heating process)
CO₂ emissions other than combustion (kg CO₂/kg)
0.1 – 0.3 kg CO₂/kg
(by-products from chemical reactions and exhaust gases)
- amount of change in resources such as soil, stone, and water (kg)
Water: 2 – 4 kg/kg (cooling, cleaning, reaction)
- input item name and input amount guideline
Alkylbenzene: 0.4 – 0.5 kg/kg (main raw material)
Sulfuric acid: 0.1 – 0.2 kg/kg (used for sulfonation reaction)
Ethylene oxide: 0.3 – 0.4 kg/kg (used for ethoxylation reaction)
Sodium hydroxide (NaOH): 0.05 – 0.1 kg/kg (used for neutralization)
- process substances such as solvents
Name: Water, alcohol
Usage: 1 – 2 kg/kg
Recirculation rate: 70 – 85% (cleaning and cooling water reuse)
- waste requiring special treatment
Name: Waste sulfuric acid, organic components in wastewater
Approximate quantity: 0.2 – 0.3 kg/kg (requires appropriate wastewater treatment)
Reference Process and Background
Manufacturing Process: Alkylbenzene is sulfonated with sulfuric acid and ethoxylated with ethylene oxide. The compound is then neutralized with sodium hydroxide to yield a compound with surfactant properties. Reuse of cooling water and treatment of waste water is important.
Environmental management: Neutralization of waste sulfuric acid and wastewater treatment are required. Use of reusable cooling water and cleaning water is recommended to reduce environmental impact.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.5 – 1.2 kWh/kg |
| Natural gas consumption | 0.02 – 0.05 kg/kg |
| CO₂ emissions other than combustion | 0.1 – 0.3 kg CO₂/kg |
| Water usage | 2 – 4 kg/kg |
| Alkylbenzene usage | 0.4 – 0.5 kg/kg |
| Sulfuric Acid Usage | 0.1 – 0.2 kg/kg |
| Ethylene oxide usage | 0.3 – 0.4 kg/kg |
| Amount of sodium hydroxide used | 0.05 – 0.1 kg/kg |
| Waste (waste sulfuric acid, wastewater) | 0.2 – 0.3 kg/kg |
Binders for polymers
(Binders for polymers (typical examples: polyvinyl alcohol (PVA), styrene-butadiene latex (SBR), etc.) are used in a wide range of applications including battery materials, paints, adhesives, and construction materials.
Input power (kWh/kg)
0.8 – 1.5 kWh/kg
(polymerization reaction, stirring, drying, filtration process)
- input fuel
Type: Natural gas
Approximate quantity:
Natural gas: 0.02 – 0.04 kg/kg (used in drying and distillation processes)
CO₂ emissions other than combustion (kg CO₂/kg)
0.1 – 0.3 kg CO₂/kg
(due to polymerization reactions and by-product emissions)
- amount of change in resources such as soil, stone, and water (kg)
Water: 3 – 6 kg/kg (cooling, cleaning, reaction)
- input item name and input amount guideline
Vinyl acetate (pre-polymerization): 0.4 – 0.5 kg/kg (for PVA-based binders)
Styrene: 0.2 – 0.3 kg/kg (for SBR binders)
Butadiene: 0.2 – 0.3 kg/kg (for SBR binders)
Ammonium persulfate: 0.01 – 0.02 kg/kg (initiator)
Sodium hydroxide (NaOH): 0.01 – 0.02 kg/kg (pH adjustment)
- process substances such as solvents
Name: Water, ethanol
Usage: 2 – 4 kg/kg
Recirculation rate: 70 – 85% (cooling and cleaning water reuse)
- waste requiring special treatment
Name: Unreacted monomers, organic components in wastewater
Approximate quantity: 0.2 – 0.4 kg/kg (proper wastewater treatment required)
Reference Process and Background
Production process: Binders for polymers are produced by polymerizing vinyl acetates or styrene butadiene. sodium hydroxide is used for pH adjustment, and reuse of cooling and cleaning water is recommended. Disposal of by-products and unreacted monomers is also important.
Environmental management: Resource efficiency must be improved through the treatment of liquid waste and the reuse of cooling water. It is also important to reduce CO₂ emissions generated from polymerization reactions.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.8 – 1.5 kWh/kg |
| Natural gas consumption | 0.02 – 0.04 kg/kg |
| CO₂ emissions other than combustion | 0.1 – 0.3 kg CO₂/kg |
| Water usage | 3 – 6 kg/kg |
| Amount of vinyl acetate used | 0.4 – 0.5 kg/kg |
| Styrene usage | 0.2 – 0.3 kg/kg |
| Butadiene usage | 0.2 – 0.3 kg/kg |
| Amount of ammonium persulfate used | 0.01 – 0.02 kg/kg |
| Amount of sodium hydroxide used | 0.01 – 0.02 kg/kg |
| Waste (unreacted materials, wastewater) | 0.2 – 0.4 kg/kg |
Polyethylene glycol (PEG)
PEG is produced by polymerizing ethylene oxide and is used in a wide range of fields, including pharmaceuticals, cosmetics, and industrial applications.
Input power (kWh/kg)
1.2 – 2.0 kWh/kg
(agitation, temperature control, filtration and drying processes)
- input fuel
Type: Natural gas
Approximate quantity:
Natural gas: 0.03 – 0.06 kg/kg (heating and drying of the reaction system)
CO₂ emissions other than combustion (kg CO₂/kg)
0.05 – 0.15 kg CO₂/kg
(emissions from polymerization reactions and by-products)
- amount of change in resources such as soil, stone, and water (kg)
Water: 1.5 – 3.0 kg/kg (used for cooling and cleaning)
- input item name and input amount guideline
Ethylene oxide (C₂H₄O): 1.0 – 1.1 kg/kg (main raw material)
Potassium hydroxide (KOH): 0.01 – 0.02 kg/kg (catalyst)
Ethanol: 0.05 – 0.1 kg/kg (impurity removal, reaction control)
- process substances such as solvents
Name: Water, ethanol
Usage: 1.5 – 3.0 kg/kg
Circulation rate: 70 – 80% (cooling water reuse)
- waste requiring special treatment
Name: Unreacted ethylene oxide, organic matter in wastewater
Approximate quantity: 0.1 – 0.3 kg/kg (requires appropriate effluent treatment)
Reference Process and Background
Production process: Ethylene oxide is polymerized in the presence of a catalyst (KOH) to produce PEG. Temperature control and appropriate pressure determine the efficiency of the polymerization reaction. Reuse of cooling water and solvent and proper treatment of unreacted materials are required.
Environmental management: Organic components in wastewater must be treated. Reuse of cooling and cleaning water is recommended to reduce environmental impact.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 1.2 – 2.0 kWh/kg |
| Natural gas consumption | 0.03 – 0.06 kg/kg |
| CO₂ emissions other than combustion | 0.05 – 0.15 kg CO₂/kg |
| Water usage | 1.5 – 3.0 kg/kg |
| Ethylene oxide usage | 1.0 – 1.1 kg/kg |
| Amount of potassium hydroxide used | 0.01 – 0.02 kg/kg |
| Ethanol usage | 0.05 – 0.1 kg/kg |
| Waste (unreacted materials, wastewater) | 0.1 – 0.3 kg/kg |
Mineral Oil
Mineral oils are refined middle distillates and heavy oils obtained mainly from crude oil distillation and refining processes, and are used as lubricants, cosmetics, and industrial oils.
Input power (kWh/kg)
0.1 – 0.3 kWh/kg
(pumping, stirring, distillation and filtration processes)
- input fuel
Type: Natural gas
Approximate quantity:
Natural gas: 0.02 – 0.05 kg/kg (for heating and distillation)
CO₂ emissions other than combustion (kg CO₂/kg)
0.01 – 0.03 kg CO₂/kg
(byproduct of the refining process)
- amount of change in resources such as soil, stone, and water (kg)
Crude oil usage: 1.5 – 2.0 kg/kg (refined feedstock)
Water: 2 – 3 kg/kg (for cooling and cleaning)
- input item name and input amount guideline
Crude oil: 1.5 – 2.0 kg/kg (main raw material)
Hydrogen: 0.01 – 0.03 kg/kg (used for desulfurization and refining)
Catalyst (alumina-based catalyst): 0.001 – 0.002 kg/kg (desulfurization catalyst)
- process substances such as solvents
Name: Water
Usage: 2 – 3 kg/kg
Recirculation rate: 75 – 85% (cooling and cleaning water reuse)
- waste requiring special treatment
Name: Waste catalyst, oil in wastewater
Approximate quantity: 0.05 – 0.1 kg/kg (proper disposal required)
Reference Process and Background
Manufacturing process: Crude oil is separated by atmospheric and vacuum distillation to produce mineral oil from middle distillates or heavy crude oil. Hydrotreating, desulfurization, and refining are performed as necessary. Reuse of cooling water and appropriate treatment of by-products contribute to reducing environmental impact.
Environmental management: Reuse of waste catalyst and removal of oil in wastewater are important.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.1 – 0.3 kWh/kg |
| Natural gas consumption | 0.02 – 0.05 kg/kg |
| CO₂ emissions other than combustion | 0.01 – 0.03 kg CO₂/kg |
| Crude oil usage | 1.5 – 2.0 kg/kg |
| Water usage | 2 – 3 kg/kg |
| Hydrogen usage | 0.01 – 0.03 kg/kg |
| Catalyst usage | 0.001 – 0.002 kg/kg |
| Waste (waste catalyst, wastewater) | 0.05 – 0.1 kg/kg |
Borate minerals
Borate ores are mined mainly in the form of borax (Na₂B₄O₇, 10H₂O)** and are obtained by washing and concentrating. Borate ores are used in the manufacture of glass, ceramics, and agricultural chemicals.
Input power (kWh/kg)
0.4 – 0.6 kWh/kg
(used in grinding, flotation, concentration, and drying processes)
- input fuel
Type: Light oil, natural gas
Approximate quantity:
Diesel oil: 0.02 – 0.04 kg/kg (for mining and hauling)
Natural gas: 0.01 – 0.02 kg/kg (drying process)
CO₂ emissions other than combustion (kg CO₂/kg)
0.01 – 0.03 kg CO₂/kg
(by-products from grinding and flotation processes)
- amount of change in resources such as soil, stone, and water (kg)
Ore throughput: 5 – 7 kg/kg (including tailings discarded as by-product)
Water: 2 – 3 kg/kg (used for washing and flotation)
- input item name and input amount guideline
Crude ore (borate ore): 5 – 7 kg/kg (main raw material)
Flocculant (polymer-based): 0.005 – 0.01 kg/kg (used in flotation process)
Slaked lime (Ca(OH)₂): 0.01 – 0.02 kg/kg (for adjusting the flotation process)
- process substances such as solvents
Name: Water
Usage: 2 – 3 kg/kg
Circulation rate: 70 – 85% (cooling and cleaning water reuse)
- waste requiring special treatment
Name: Tailings, flotation waste
Approximate quantity: 4 – 6 kg/kg (contains unused minerals and by-products)
Reference Process and Background
Manufacturing Process: Mined coarse ore is crushed, flotation and dried, and then concentrated as borate ore. Since a lot of water is used, reuse is important to reduce environmental impact. Tailings and flotation waste are generated as waste and must be managed appropriately.
Environmental management: Tailings and flotation effluent are managed at the processing facility to reduce environmental impact.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.4 – 0.6 kWh/kg |
| Diesel oil consumption | 0.02 – 0.04 kg/kg |
| Natural gas consumption | 0.01 – 0.02 kg/kg |
| CO₂ emissions other than combustion | 0.01 – 0.03 kg CO₂/kg |
| Ore processing volume | 5 – 7 kg/kg |
| Water usage | 2 – 3 kg/kg |
| Amount of coagulant used | 0.005 – 0.01 kg/kg |
| Amount of slaked lime used | 0.01 – 0.02 kg/kg |
Acid/alkali
Below are typical acids and alkalis commonly used in refining processes and their applications and approximate share of use in the industrial sector. These substances are used in applications as diverse as chemical manufacturing, petroleum refining, and electronic component cleaning.
- typical acids used in purification and their shares
| Name of acid | use | Share (%) |
| Sulfuric acid (H₂SO₄) | Petroleum refining, mineral processing, chemical manufacturing | 40 – 50%. |
| Hydrochloric acid (HCl) | Metal cleaning, pickling, electronic component cleaning | 20 – 30 |
| Nitric acid (HNO₃) | Metalworking, electronic component manufacturing, chemical synthesis | 10 – 20%. |
| Phosphoric acid (H₃PO₄) | Food, electronics industry, surface treatment | 5 – 10% |
| Acetic acid (CH₃COOH) | Pharmaceuticals, chemicals, food processing | 5 – 10% |
Acid Applications and Selection Criteria
Sulfuric Acid: The most versatile acid, used in petroleum refining, mineral leaching, and battery production.
Hydrochloric acid: Acid pickling removes oxides from metal surfaces and is also used in oil drilling.
Nitric acid: Used for etching electronic components and manufacturing pharmaceuticals.
Phosphoric acid: Used as a food additive and for cleaning electronic components.
Acetic acid: Used as a raw material or solvent in chemical synthesis.
- typical alkalis used in purification and their share
| Name of Alkali | use | Share (%) |
| Sodium hydroxide (NaOH) | Pulp and paper, textiles, wastewater treatment, chemical manufacturing | 60 – 70%. |
| Potassium hydroxide (KOH) | Chemical manufacturing, batteries, pharmaceuticals | 10 – 15 |
| Sodium carbonate (Na₂CO₃) | Glass production, chemical synthesis | 10 – 15% of |
| Ammonia (NH₃) | Coolant, wastewater treatment, fertilizer production | 5 – 10% |
Alkali Applications and Selection Criteria
Sodium hydroxide (NaOH): The most commonly used alkali in refining processes, it is active in the chemical, pulp and paper, and textile industries.
Potassium hydroxide (KOH): As a high-quality alkali, it is used in the manufacture of batteries, pharmaceuticals, and specialty chemicals.
Sodium carbonate (Na₂CO₃): used in glassmaking and chemical processes.
Ammonia (NH₃): used as a refrigerant, wastewater treatment, and fertilizer.
summary
Below is a list of representative shares of acids and alkalis for refining:
Acid Share
Sulfuric acid: 40 – 50%.
Hydrochloric acid: 20 – 30
Nitric acid: 10 – 20%.
Phosphoric Acid: 5 – 10%
Acetic Acid: 5 – 10%
Alkali Share
Sodium hydroxide: 60 – 70%.
Potassium hydroxide: 10 – 15%.
Sodium carbonate: 10 – 15%.
Ammonia: 5 – 10%
These acids and alkalis are selected according to their applications and play an important role in processes such as refining, surface treatment, and wastewater treatment, among others.
(food) starch
Starch is extracted primarily from plants such as corn, potatoes, and cassava and is used in the manufacture of food and industrial products. Typical manufacturing processes include grinding, separating, and drying of plant materials.
Input power (kWh/kg)
0.4 – 0.6 kWh/kg
(used in grinding, separation, stirring, and drying processes)
- input fuel
Type: Natural gas
Approximate quantity:
Natural gas: 0.02 – 0.05 kg/kg (used in drying process)
CO₂ emissions other than combustion (kg CO₂/kg)
0.01 – 0.03 kg CO₂/kg
(by-product of grinding and separation processes)
- amount of change in resources such as soil, stone, and water (kg)
Water: 3 – 5 kg/kg (used in washing and separation processes)
- input item name and input amount guideline
Corn or other raw material: 1.2 – 1.5 kg/kg (main raw material)
Sulfuric acid: 0.005 – 0.01 kg/kg (used for pH adjustment)
Sodium hydroxide (NaOH): 0.005 – 0.01 kg/kg (used to neutralize pH)
- process substances such as solvents
Name: Water
Usage: 3 – 5 kg/kg
Recirculation rate: 70 – 80% (cleaning and cooling water reuse)
- waste requiring special treatment
Name: Organic components in unused residues and wastewater
Approximate quantity: 0.2 – 0.4 kg/kg (requires proper treatment of unused portions and liquid waste)
Reference Process and Background
Manufacturing Process: Corn and other raw materials are crushed and separated into starch and by-products (fiber and protein). The starch produced is dried and shipped as the final product. Washing water and cooling water are often reused, and waste management is also important.
Environmental Management: It is recommended that unused residues be reused as feedstock for animal feed or biogas. Organic components in wastewater must be treated appropriately.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.4 – 0.6 kWh/kg |
| Natural gas consumption | 0.02 – 0.05 kg/kg |
| CO₂ emissions other than combustion | 0.01 – 0.03 kg CO₂/kg |
| Water usage | 3 – 5 kg/kg |
| Amount of raw materials (corn, etc.) used | 1.2 – 1.5 kg/kg |
| Sulfuric Acid Usage | 0.005 – 0.01 kg/kg |
| Amount of sodium hydroxide used | 0.005 – 0.01 kg/kg |
| Waste (residues, wastewater) | 0.2 – 0.4 kg/kg |
Ethylenedichloride (EDC, C₂H₄Cl₂)
EDC is produced by direct chlorination of ethylene and chlorine or oxidative chlorination of hydrogen chloride and ethylene. edc is used as a raw material for vinyl chloride monomer (VCM)** and plays a key role in the production of vinyl chloride resin (PVC).
Input power (kWh/kg)
0.6 – 1.2 kWh/kg
(reaction temperature maintenance, stirring, distillation and filtration processes)
- input fuel
Type: Natural gas
Approximate quantity:
Natural gas: 0.02 – 0.05 kg/kg (used in distillation and drying processes)
CO₂ emissions other than combustion (kg CO₂/kg)
0.05 – 0.1 kg CO₂/kg
(byproduct of the chlorination oxidation process)
- amount of change in resources such as soil, stone, and water (kg)
Water: 3 – 4 kg/kg (for cooling and cleaning)
- input item name and input amount guideline
Ethylene (C₂H₄): 0.4 – 0.6 kg/kg (main raw material)
Chlorine (Cl₂): 0.6 – 0.8 kg/kg (direct chlorination process)
Hydrogen chloride (HCl): 0.1 – 0.2 kg/kg (chlorination oxidation process)
Catalysts (CuCl₂, KCl): 0.005 – 0.01 kg/kg (used for chlorination oxidation)
- process substances such as solvents
Name: Water
Usage: 3 – 4 kg/kg
Recirculation rate: 80 – 90% (cooling and cleaning water reuse)
- waste requiring special treatment
Name: Chlorinated effluents, byproducts (e.g., isomers of dichloroethane)
Approximate quantity: 0.1 – 0.2 kg/kg (proper treatment of effluent required)
Reference Process and Background
Manufacturing Process:
Direct chlorination: EDC is produced by reacting ethylene with chlorine. Purification is required to remove chlorinated isomers as byproducts.
Chlorination Oxidation: Ethylene, hydrogen chloride, and oxygen react to produce EDC in the presence of a catalyst. This process is economical in that excess HCl can be reused.
Environmental Management: Reuse of cooling water is important for resource efficiency. In addition, treatment of waste chlorinated liquid waste is necessary for environmental protection.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.6 – 1.2 kWh/kg |
| Natural gas consumption | 0.02 – 0.05 kg/kg |
| CO₂ emissions other than combustion | 0.05 – 0.1 kg CO₂/kg |
| Water usage | 3 – 4 kg/kg |
| Ethylene usage | 0.4 – 0.6 kg/kg |
| Chlorine usage | 0.6 – 0.8 kg/kg |
| Hydrogen chloride usage | 0.1 – 0.2 kg/kg |
| Catalyst usage | 0.005 – 0.01 kg/kg |
| Waste (Chlorinated effluent) | 0.1 – 0.2 kg/kg |
Castor Oil
Castor oil is obtained from the seeds of the castor (caster) plant and is used in lubricants, pharmaceuticals, cosmetics, and resin manufacturing. It is produced by pressing or solvent extraction.
Input power (kWh/kg)
0.5 – 0.8 kWh/kg
(used for hydraulic pressing, agitation, dewatering, and filtration processes)
- input fuel
Type: Natural gas
Approximate quantity:
Natural gas: 0.01 – 0.03 kg/kg (used for drying process)
CO₂ emissions other than combustion (kg CO₂/kg)
0.02 – 0.05 kg CO₂/kg
(byproduct of solvent treatment and transport)
- amount of change in resources such as soil, stone, and water (kg)
Water: 1 – 2 kg/kg (for cooling and cleaning)
Castor seed husk/waste: 3 – 4 kg/kg (residue after pressing)
- input item name and input amount guideline
Castor seed: 2.5 – 3.5 kg/kg (main raw material)
Solvent (hexane): 0.05 – 0.1 kg/kg (for solvent extraction method)
Catalyst (aluminum oxide): 0.01 – 0.02 kg/kg (for dewatering process)
Degradation rate: 10 – 15%/year (periodic catalyst replacement required)
- process substances such as solvents
Name: Hexane, water
Usage: 1 – 2 kg/kg
Recirculation rate: 80 – 90% (solvent and water reuse)
- waste requiring special treatment
Name: Oil residues, solvent waste
Approximate quantity: 0.3 – 0.5 kg/kg (pressed oil residue can be reused as feed)
Reference Process and Background
Manufacturing Process:
Pressing: Castor seeds are crushed and the oil is extracted by mechanical pressing.
Solvent extraction method: Oil not obtained by pressing is extracted with hexane and the solvent is recovered by distillation.
Environmental management: Reuse of solvents and recycling of pressing residues are important. Periodic replacement of catalysts is required.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.5 – 0.8 kWh/kg |
| Natural gas consumption | 0.01 – 0.03 kg/kg |
| CO₂ emissions other than combustion | 0.02 – 0.05 kg CO₂/kg |
| Water usage | 1 – 2 kg/kg |
| Castor Seed Usage | 2.5 – 3.5 kg/kg |
| Solvent (hexane) | 0.05 – 0.1 kg/kg |
| Catalyst (aluminum oxide) | 0.01 – 0.02 kg/kg |
| Catalyst degradation rate | 10 – 15%/year |
| Waste (residues, effluents) | 0.3 – 0.5 kg/kg |
Hindered Phenol
Hindered phenols are mainly used as antioxidants to stabilize resins, rubbers, and lubricants. A typical production process is the alkylation reaction of phenol.
Input power (kWh/kg)
1.5 – 2.5 kWh/kg
(agitation, temperature control, filtration, drying process)
- input fuel
Type: Natural gas
Approximate quantity:
Natural gas: 0.02 – 0.04 kg/kg (heating process)
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.05 – 0.1 kg CO₂/kg
(emissions from chemical reactions and by-products)
- amount of change in resources such as soil, stone, and water (kg)
Water: 2 – 3 kg/kg (for cooling and cleaning)
- input item name and input amount guideline
Phenol: 0.6 – 0.8 kg/kg (main reactant)
Alkylating agent (e.g., isobutylene): 0.4 – 0.5 kg/kg
Acid catalyst (e.g. phosphoric acid catalyst): 0.01 – 0.02 kg/kg
Degradation rate: 15 – 20%/kg (constant amount of degradation per reaction)
- process substances such as solvents
Name: Toluene, Ethanol
Usage: 0.2 – 0.4 kg/kg
Circulation rate: 70 – 85% (solvent reuse)
- waste requiring special treatment
Name: Unreacted phenol, byproducts (polymeric byproducts)
Approximate quantity: 0.05 – 0.1 kg/kg (requires appropriate effluent treatment)
Reference Process and Background
Manufacturing Process:
Phenol and an alkylating agent (e.g., isobutylene) react in the presence of an acidic catalyst to produce hindered phenol. Control of reaction temperature is important, and elimination of byproducts is necessary.
Environmental management: Proper treatment of unreacted phenol and polymer byproducts is required. Resource efficiency can also be improved by promoting solvent reuse.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 1.5 – 2.5 kWh/kg |
| Natural gas consumption | 0.02 – 0.04 kg/kg |
| CO₂ emissions other than combustion | 0.05 – 0.1 kg CO₂/kg |
| Water usage | 2 – 3 kg/kg |
| Phenol usage | 0.6 – 0.8 kg/kg |
| Amount of alkylating agent used | 0.4 – 0.5 kg/kg |
| Catalyst (acid catalyst) | 0.01 – 0.02 kg/kg |
| Catalyst degradation rate | 15 – 20%/kg |
| Solvent usage | 0.2 – 0.4 kg/kg |
| Waste (byproducts, unreacted materials) | 0.05 – 0.1 kg/kg |
Phosphate Ester
Phosphate esters are used as surfactants, plasticizers, lubricants, or flame retardants. The most common production process is an esterification reaction using alcohol and phosphoric acid or phosphoric anhydride.
Input power (kWh/kg)
1.0 – 2.0 kWh/kg
(agitation, temperature control, filtration and drying processes)
- input fuel
Type: Natural gas
Approximate quantity:
Natural gas: 0.03 – 0.05 kg/kg (heating process)
CO₂ emissions other than combustion (kg CO₂/kg)
0.02 – 0.05 kg CO₂/kg
(emissions from by-products and reaction gases)
- amount of change in resources such as soil, stone, and water (kg)
Water: 2 – 4 kg/kg (for cooling and cleaning)
- input item name and input amount guideline
Phosphoric anhydride (P₂O₅): 0.3 – 0.4 kg/kg (main reactant)
Alcohol (ethanol, butanol): 0.7 – 0.8 kg/kg
Acid catalyst (sulfuric or phosphoric acid): 0.01 – 0.02 kg/kg
Degradation rate: 10 – 15%/kg (degradation due to by-product accumulation)
- process substances such as solvents
Name: Toluene, water
Usage: 0.2 – 0.5 kg/kg
Recirculation rate: 75 – 85% (solvent and cooling water reuse)
- waste requiring special treatment
Name: Unreacted alcohol, acidic wastewater
Approximate quantity: 0.1 – 0.3 kg/kg (effluent requires neutralization treatment)
Reference Process and Background
Production process: Phosphoric anhydride and alcohol are reacted by esterification to produce phosphate esters. An acidic catalyst is used to increase the reaction rate, and the effluent after the process requires appropriate neutralization treatment.
Environmental management: Proper disposal of unreacted alcohol and waste acid is required, and reuse of solvents and cooling water contributes to resource efficiency.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 1.0 – 2.0 kWh/kg |
| Natural gas consumption | 0.03 – 0.05 kg/kg |
| CO₂ emissions other than combustion | 0.02 – 0.05 kg CO₂/kg |
| Water usage | 2 – 4 kg/kg |
| Amount of phosphoric anhydride used | 0.3 – 0.4 kg/kg |
| Alcohol usage | 0.7 – 0.8 kg/kg |
| Catalyst (acid catalyst) | 0.01 – 0.02 kg/kg |
| Catalyst degradation rate | 10 – 15%/kg |
| Solvent usage | 0.2 – 0.5 kg/kg |
| Waste (unreacted materials, waste acid) | 0.1 – 0.3 kg/kg |
Acrylonitrile (Acrylonitrile, CH₂=CH-CN)
Acrylonitrile is mainly used in the production of styrene-acrylonitrile (SAN) resin and polyacrylonitrile (PAN), with the typical production method being the ammo-oxidation process of propylene.
Input power (kWh/kg)
1.8 – 2.5 kWh/kg
(agitation, temperature and pressure control, filtration and drying)
- input fuel
Type: Natural gas
Approximate quantity:
Natural gas: 0.02 – 0.04 kg/kg (heating and drying of reaction system)
CO₂ emissions other than combustion (kg CO₂/kg)
0.1 – 0.15 kg CO₂/kg
(emissions from chemical reactions and by-products)
- amount of change in resources such as soil, stone, and water (kg)
Water: 2.5 – 4 kg/kg (cooling, cleaning)
- input item name and input amount guideline
Propylene (C₃H₆): 0.6 – 0.8 kg/kg (main raw material)
Ammonia (NH₃): 0.2 – 0.3 kg/kg (for ammonia oxidation reaction)
Oxygen (or air-derived): supply required
Catalyst (bismuth-molybdenum oxide): 0.01 – 0.02 kg/kg
Degradation rate: 2 – 5%/kg (catalyst is periodically replaced because it degrades at high temperatures)
- process substances such as solvents
Name: Water
Usage: 2.5 – 4 kg/kg
Circulation rate: 75 – 85% (cooling and cleaning water reuse)
- waste requiring special treatment
Name: Unreacted propylene, byproducts (acetonitrile, waste liquid containing cyanide)
Approximate quantity: 0.2 – 0.3 kg/kg (proper handling required)
Reference Process and Background
Manufacturing Process:
Propylene, ammonia, and oxygen react under high temperature conditions in the presence of a catalyst to produce acrylonitrile. Since acetonitrile and hydrogen cyanide are generated as byproducts, effluent treatment is important.
Recovery and reuse of cooling water and unreacted gas is important for resource efficiency.
Environmental management: Waste liquid containing cyanide and acetonitrile must be treated.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 1.8 – 2.5 kWh/kg |
| Natural gas consumption | 0.02 – 0.04 kg/kg |
| CO₂ emissions other than combustion | 0.1 – 0.15 kg CO₂/kg |
| Water usage | 2.5 – 4 kg/kg |
| Propylene usage | 0.6 – 0.8 kg/kg |
| Ammonia usage | 0.2 – 0.3 kg/kg |
| Catalyst usage | 0.01 – 0.02 kg/kg |
| Catalyst degradation rate | 2 – 5%/kg |
| Solvent usage | 2.5 – 4 kg/kg |
| Waste (byproducts, effluents) | 0.2 – 0.3 kg/kg |
Metal Acrylate
Metal acrylates are obtained by the reaction of acrylic acid with metal salts and are used as paints, adhesives, and surface coating agents.
Input power (kWh/kg)
1.2 – 2.0 kWh/kg
(agitation, temperature control, filtration and drying processes)
- input fuel
Type: Natural gas
Approximate quantity:
Natural gas: 0.02 – 0.04 kg/kg (used for heating and drying reaction systems)
CO₂ emissions other than combustion (kg CO₂/kg)
0.03 – 0.06 kg CO₂/kg
(emissions from chemical reactions and by-products)
- amount of change in resources such as soil, stone, and water (kg)
Water: 3 – 5 kg/kg (used for cooling and cleaning)
- input item name and input amount guideline
Acrylic acid (CH₂=CH-COOH): 0.6 – 0.7 kg/kg (main raw material)
Metal salt (e.g., zinc salt ZnO or calcium salt Ca(OH)₂): 0.3 – 0.4 kg/kg
Catalyst (acid or basic catalyst): 0.01 – 0.02 kg/kg
Degradation rate: 10 – 15%/kg (reaction degradation at high temperature)
- process substances such as solvents
Name: Water, ethanol
Usage: 2 – 3 kg/kg
Circulation rate: 80 – 90% (reuse of cooling water and solvents)
- waste requiring special treatment
Name: Unreacted acrylic acid, byproduct (polymerized product)
Approximate quantity: 0.1 – 0.2 kg/kg (unreacted material and liquid waste)
Reference Process and Background
Manufacturing Process:
Acrylic acid and metal salts are mixed and reacted in the presence of a catalyst to produce metal acrylate. The reaction requires proper temperature control and the recovery and reuse of unreacted materials.
Environmental control: Controls are needed to prevent polymerization of byproducts, and reuse of cooling water and ethanol is recommended.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 1.2 – 2.0 kWh/kg |
| Natural gas consumption | 0.02 – 0.04 kg/kg |
| CO₂ emissions other than combustion | 0.03 – 0.06 kg CO₂/kg |
| Water usage | 3 – 5 kg/kg |
| Amount of acrylic acid used | 0.6 – 0.7 kg/kg |
| Metal salt usage | 0.3 – 0.4 kg/kg |
| Catalyst usage | 0.01 – 0.02 kg/kg |
| Catalyst degradation rate | 10 – 15%/kg |
| Solvent usage | 2 – 3 kg/kg |
| Waste (unreacted materials, byproducts) | 0.1 – 0.2 kg/kg |
Potassium persulfate (K₂S₂O₈)
Potassium persulfate is used as an oxidizing agent and initiator in the manufacture of synthetic resins and electronic components. A typical production process is electrolytic oxidation of potassium sulfate and ammonium sulfate.
Input power (kWh/kg)
2.5 – 3.5 kWh/kg
(electrolysis reaction in electrolyzer, agitation, filtration, drying)
- input fuel
Type: Natural gas
Approximate quantity:
Natural gas: 0.03 – 0.05 kg/kg (drying process)
CO₂ emissions other than combustion (kg CO₂/kg)
0.01 – 0.02 kg CO₂/kg
(byproduct from process)
- amount of change in resources such as soil, stone, and water (kg)
Water: 4 – 6 kg/kg (as electrolysis and cooling water)
- input item name and input amount guideline
Potassium sulfate (K₂SO₄): 0.7 – 0.8 kg/kg (main raw material)
Sulfuric acid (H₂SO₄): 0.2 – 0.3 kg/kg (auxiliary component of electrolyte)
Catalyst (e.g. cobalt oxide): 0.01 – 0.02 kg/kg
Degradation rate: 10 – 15%/kg (reaction degradation in electrolytic cell)
- process substances such as solvents
Name: Water
Usage: 4 – 6 kg/kg
Recirculation rate: 80 – 90% (cooling water and process water reuse)
- waste requiring special treatment
Name: Waste liquids containing sulfuric acid, byproducts (e.g., ammonium sulfate)
Approximate quantity: 0.2 – 0.3 kg/kg (neutralization treatment of effluent required)
Reference Process and Background
Manufacturing Process:
Potassium sulfate is oxidized in an electrolyzer to produce potassium persulfate. Sulfuric acid is used as an auxiliary electrolyte, and high voltage is required in the electrolyzer.
Environmental management: By-products of the process, such as ammonium sulfate and waste acid, are neutralized or recycled.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 2.5 – 3.5 kWh/kg |
| Natural gas consumption | 0.03 – 0.05 kg/kg |
| CO₂ emissions other than combustion | 0.01 – 0.02 kg CO₂/kg |
| Water usage | 4 – 6 kg/kg |
| Amount of potassium sulfate used | 0.7 – 0.8 kg/kg |
| Sulfuric Acid Usage | 0.2 – 0.3 kg/kg |
| Catalyst usage | 0.01 – 0.02 kg/kg |
| Catalyst degradation rate | 10 – 15%/kg |
| Solvent usage | 4 – 6 kg/kg |
| Waste (effluents, byproducts) | 0.2 – 0.3 kg/kg |
Polyvinyl alcohol (PVA)
PVA is widely used in adhesives, films, fibers, and paper processing. The typical production process is polymerization of vinyl acetate monomer (VAM) and subsequent alcoholysis.
Input power (kWh/kg)
1.5 – 2.5 kWh/kg
(polymerization, reaction temperature control, filtration and drying processes)
- input fuel
Type: Natural gas
Approximate quantity:
Natural gas: 0.03 – 0.06 kg/kg (used for heating and drying)
CO₂ emissions other than combustion (kg CO₂/kg)
0.05 – 0.1 kg CO₂/kg
(during reaction and by-product emissions)
- amount of change in resources such as soil, stone, and water (kg)
Water: 4 – 6 kg/kg (for cooling and cleaning)
- input item name and input amount guideline
Vinyl acetate monomer (VAM): 1.1 – 1.2 kg/kg (main raw material)
Methanol: 0.1 – 0.2 kg/kg (to accelerate alcohol degradation)
Acid or base catalyst (NaOH or H₂SO₄): 0.01 – 0.02 kg/kg
Degradation rate: 5 – 10%/kg (replacement frequency relative to catalyst usage)
- process substances such as solvents
Name: Methanol, water
Usage: 2 – 4 kg/kg
Recirculation rate: 80 – 90% (solvent and cooling water reuse)
- waste requiring special treatment
Name: Unreacted VAM, effluent (acidic or alkaline)
Approximate quantity: 0.1 – 0.2 kg/kg (requires appropriate effluent treatment)
Reference Process and Background
Manufacturing Process:
Polyvinyl acetate monomer is polymerized to obtain polyvinyl acetate, which is then alcohol decomposed to produce PVA. During the process, the reuse of cooling water is critical.
Environmental management: Unreacted vinyl acetate and liquid waste must be disposed of properly to reduce environmental impact.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 1.5 – 2.5 kWh/kg |
| Natural gas consumption | 0.03 – 0.06 kg/kg |
| CO₂ emissions other than combustion | 0.05 – 0.1 kg CO₂/kg |
| Water usage | 4 – 6 kg/kg |
| Amount of vinyl acetate used | 1.1 – 1.2 kg/kg |
| Methanol usage | 0.1 – 0.2 kg/kg |
| Catalyst usage | 0.01 – 0.02 kg/kg |
| Catalyst degradation rate | 5 – 10%/kg |
| Solvent usage | 2 – 4 kg/kg |
| Waste (unreacted materials, liquid waste) | 0.1 – 0.2 kg/kg |
Benzoyl Chloride, C₇H₅ClO
Benzoyl chlorides are widely used as intermediates in dyes, pharmaceuticals, agrochemicals, and fragrances. Typical production methods are oxidation of benzotrichloride or chlorination of benzoic acid.
Input power (kWh/kg)
1.0 – 2.0 kWh/kg
(agitation, temperature control, filtration and drying)
- input fuel
Type: Natural gas
Approximate quantity:
Natural gas: 0.03 – 0.05 kg/kg (used for heating and drying processes)
CO₂ emissions other than combustion (kg CO₂/kg)
0.05 – 0.08 kg CO₂/kg
(from chemical reactions and by-products)
- amount of change in resources such as soil, stone, and water (kg)
Water: 3 – 4 kg/kg (cooling and cleaning)
- input item name and input amount guideline
Benzoic acid (C₇H₆O₂): 0.8 – 1.0 kg/kg (main raw material)
Chlorine (Cl₂): 0.4 – 0.5 kg/kg (used for chlorination)
Catalyst (iron chloride such as FeCl₃): 0.01 – 0.02 kg/kg
Degradation rate: 5 – 10%/kg (gradual deactivation of catalyst)
- process substances such as solvents
Name: Dichloromethane (DCM) or Toluene
Usage: 1 – 2 kg/kg
Recirculation rate: 80 – 90% (solvent reuse)
- waste requiring special treatment
Name: Byproducts (HCl gas, unreacted benzoic acid), solvent waste
Approximate amount: 0.1 – 0.3 kg/kg (HCl gas requires neutralization treatment)
Reference Process and Background
Manufacturing Process:
Benzoic acid and chlorine react in the presence of an iron chloride catalyst to obtain benzoyl chloride. During the reaction, HCl is formed as a byproduct, so neutralization treatment is necessary.
Cooling and solvent reuse improve resource efficiency.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 1.0 – 2.0 kWh/kg |
| Natural gas consumption | 0.03 – 0.05 kg/kg |
| CO₂ emissions other than combustion | 0.05 – 0.08 kg CO₂/kg |
| Water usage | 3 – 4 kg/kg |
| Amount of benzoic acid used | 0.8 – 1.0 kg/kg |
| Chlorine usage | 0.4 – 0.5 kg/kg |
| Catalyst usage | 0.01 – 0.02 kg/kg |
| Catalyst degradation rate | 5 – 10%/kg |
| Solvent usage | 1 – 2 kg/kg |
| Waste (byproducts, effluents) | 0.1 – 0.3 kg/kg |
Calcium chloride (CaCl₂)
Calcium chloride is widely used for road anti-icing, desiccants, dehumidifiers, and industrial applications. The typical production method is by a neutralization reaction of calcium carbonate and hydrochloric acid.
Input power (kWh/kg)
0.6 – 1.2 kWh/kg
(agitation, drying, filtration process)
- input fuel
Type: Natural gas
Approximate quantity:
Natural gas: 0.05 – 0.08 kg/kg (heating and drying process)
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.3 – 0.5 kg CO₂/kg
(CO₂ release from calcium carbonate)
- amount of change in resources such as soil, stone, and water (kg)
Water: 2 – 3 kg/kg (used for reaction, cooling, washing)
- input item name and input amount guideline
Calcium carbonate (CaCO₃): 0.7 – 0.8 kg/kg (main raw material)
Hydrochloric acid (HCl, 37%): 0.4 – 0.5 kg/kg (for acid reaction)
Catalyst (not used): No catalyst is typically used in this process.
- process substances such as solvents
Name: Water
Usage: 2 – 3 kg/kg
Circulation rate: 75 – 85% (cooling and cleaning water reuse)
- waste requiring special treatment
Name: Waste acid, unreacted calcium carbonate
Approximate amount: 0.1 – 0.2 kg/kg (waste acid requires neutralization treatment)
Reference Process and Background
Production process: Calcium chloride and carbon dioxide are produced by the reaction of calcium carbonate and hydrochloric acid. After washing the reaction products, calcium chloride is concentrated and dried to make the product.
Environmental management: CO₂ emissions and proper treatment of waste acid are necessary. In addition, reuse of cleaning water improves resource efficiency.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.6 – 1.2 kWh/kg |
| Natural gas consumption | 0.05 – 0.08 kg/kg |
| CO₂ emissions other than combustion | 0.3 – 0.5 kg CO₂/kg |
| Water usage | 2 – 3 kg/kg |
| Amount of calcium carbonate used | 0.7 – 0.8 kg/kg |
| Hydrochloric acid usage | 0.4 – 0.5 kg/kg |
| Solvent usage | 2 – 3 kg/kg |
| Waste (waste acid, unreacted materials) | 0.1 – 0.2 kg/kg |
Molybdenum oxide (MoO₃)
Molybdenum oxide is widely used in alloy additives, catalysts, and chemical processes and is produced primarily from ore (molybdenum concentrate).
Input power (kWh/kg)
2.0 – 3.5 kWh/kg
(grinding, firing, temperature control and filtration processes)
- input fuel
Type: Natural gas
Approximate quantity:
Natural gas: 0.04 – 0.06 kg/kg (firing and drying process)
CO₂ emissions other than combustion (kg CO₂/kg)
0.02 – 0.05 kg CO₂/kg
(processing and by-products of molybdenum concentrates)
- amount of change in resources such as soil, stone, and water (kg)
Water: 2 – 4 kg/kg (washing and cooling)
- input item name and input amount guideline
Molybdenum concentrate (MoS₂): 1.5 – 2.0 kg/kg (main raw material)
Oxygen (O₂): 0.2 – 0.3 kg/kg (oxidation reaction)
Catalyst (barium oxide BaO): 0.01 – 0.02 kg/kg
Degradation rate: 10 – 15%/kg (degradation due to repeated use)
- process substances such as solvents
Name: Water
Usage: 2 – 4 kg/kg
Circulation rate: 75 – 85% (cooling and cleaning water reuse)
- waste requiring special treatment
Name: Sulfide by-products, unreacted concentrates
Approximate quantity: 0.2 – 0.3 kg/kg (byproducts require appropriate treatment)
Reference Process and Background
Manufacturing Process:
Molybdenum concentrate (MoS₂) is roasted and reacted with oxygen to produce molybdenum oxide (MoO₃). Since sulfur compounds are generated as byproducts in the calcination process, appropriate waste treatment is required.
Environmental management: Reuse of cooling water contributes to resource efficiency, and management of byproducts is important to reduce environmental impact.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 2.0 – 3.5 kWh/kg |
| Natural gas consumption | 0.04 – 0.06 kg/kg |
| CO₂ emissions other than combustion | 0.02 – 0.05 kg CO₂/kg |
| Water usage | 2 – 4 kg/kg |
| Molybdenum concentrate usage | 1.5 – 2.0 kg/kg |
| Oxygen usage | 0.2 – 0.3 kg/kg |
| Catalyst usage | 0.01 – 0.02 kg/kg |
| Catalyst degradation rate | 10 – 15%/kg |
| Waste (sulfide, byproducts) | 0.2 – 0.3 kg/kg |
Cobalt nitrate (Co(NO₃)₂)
Cobalt nitrate is used in applications such as catalysts, battery materials, and dyes. The reaction of cobalt metal or cobalt oxide with nitric acid is commonly used for production.
Input power (kWh/kg)
0.8 – 1.5 kWh/kg
(reaction temperature control, stirring, filtration, drying)
- input fuel
Type: Natural gas
Approximate quantity:
Natural gas: 0.02 – 0.04 kg/kg (heating and drying process)
CO₂ emissions other than combustion (kg CO₂/kg)
0.03 – 0.06 kg CO₂/kg
(emissions from process and by-products)
- amount of change in resources such as soil, stone, and water (kg)
Water: 3 – 5 kg/kg (washing and cooling)
- input item name and input amount guideline
Cobalt metal (Co): 0.3 – 0.5 kg/kg (main raw material)
Nitric acid (HNO₃): 0.6 – 0.8 kg/kg (for oxidation and dissolution)
Catalyst (not used): No catalyst is used in the general process.
- process substances such as solvents
Name: Water
Usage: 3 – 5 kg/kg
Recirculation rate: 80 – 90% (cooling and cleaning water reuse)
- waste requiring special treatment
Name: Waste acid, unreacted cobalt
Approximate amount: 0.1 – 0.2 kg/kg (requires appropriate neutralization treatment)
Reference Process and Background
Manufacturing Process:
Cobalt metal is reacted with nitric acid to produce cobalt nitrate. The reaction temperature and stirring rate are controlled, and the product is filtered and dried.
Environmental management: Proper treatment of unreacted materials and waste acid is necessary. In addition, water reuse improves resource efficiency.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.8 – 1.5 kWh/kg |
| Natural gas consumption | 0.02 – 0.04 kg/kg |
| CO₂ emissions other than combustion | 0.03 – 0.06 kg CO₂/kg |
| Water usage | 3 – 5 kg/kg |
| Cobalt metal usage | 0.3 – 0.5 kg/kg |
| Nitric acid usage | 0.6 – 0.8 kg/kg |
| Solvent usage | 3 – 5 kg/kg |
| Waste (waste acid, unreacted materials) | 0.1 – 0.2 kg/kg |
Barium oxide (BaO)
Barium oxide is used in catalysts, glassmaking, and chemical processes, and is produced primarily from barium ore (deuterite BaSO₄) or barium carbonate (BaCO₃)**.
Input power (kWh/kg)
1.5 – 2.5 kWh/kg
(firing temperature control, agitation, grinding, filtration)
- input fuel
Type: Natural gas
Approximate quantity:
Natural gas: 0.05 – 0.08 kg/kg (firing process)
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.3 – 0.4 kg CO₂/kg
(decomposition gas from barium carbonate)
- amount of change in resources such as soil, stone, and water (kg)
Water: 2 – 3 kg/kg (for cleaning and cooling)
- input item name and input amount guideline
Barium carbonate (BaCO₃): 1.5 – 1.8 kg/kg (main raw material)
Oxygen: used as a reaction from supplied air
Catalyst (not used): No catalyst is typically used in this process.
- process substances such as solvents
Name: Water
Usage: 2 – 3 kg/kg
Circulation rate: 75 – 85% (cooling and cleaning water reuse)
- waste requiring special treatment
Name: Byproducts (unreacted BaCO₃, trace sulfides)
Approximate quantity: 0.1 – 0.2 kg/kg (requires processing)
Reference Process and Background
Manufacturing Process:
Barium carbonate (BaCO₃) is calcined at high temperatures (>1000°C) to produce barium oxide (BaO) and carbon dioxide. Temperature control is important, and unreacted barium carbonate is generally reused.
Environmental management: Process management to reduce CO₂ emissions and reuse of cooling water contribute to resource efficiency.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 1.5 – 2.5 kWh/kg |
| Natural gas consumption | 0.05 – 0.08 kg/kg |
| CO₂ emissions other than combustion | 0.3 – 0.4 kg CO₂/kg |
| Water usage | 2 – 3 kg/kg |
| Amount of barium carbonate used | 1.5 – 1.8 kg/kg |
| Solvent usage | 2 – 3 kg/kg |
| Waste (unreacted materials, byproducts) | 0.1 – 0.2 kg/kg |
p-Hydroxybenzoic acid (PHBA)
PHBA is used in **polymer precursors (especially liquid crystal polymers)**, pharmaceuticals, and cosmetics. Industrial methods include the oxidation of paraformaldehyde with potassium salts.
Input power (kWh/kg)
1.8 – 2.5 kWh/kg
(agitation, temperature control, filtration and drying)
- input fuel
Type: Natural gas
Approximate quantity:
Natural gas: 0.03 – 0.05 kg/kg (heating and drying process)
CO₂ emissions other than combustion (kg CO₂/kg)
0.05 – 0.08 kg CO₂/kg
(byproduct emissions during process)
- amount of change in resources such as soil, stone, and water (kg)
Water: 3 – 5 kg/kg (cooling and cleaning)
- input item name and input amount guideline
Phenol (C₆H₅OH): 0.6 – 0.7 kg/kg (main raw material)
Carbon dioxide (CO₂): 0.2 – 0.3 kg/kg (for carboxylation)
Catalyst (copper oxide or basic catalyst): 0.01 – 0.02 kg/kg
Degradation rate: 10 – 15%/kg (degrades at high temperatures)
- process substances such as solvents
Name: Water, toluene (or similar solvent)
Usage: 2 – 4 kg/kg
Recirculation rate: 80 – 90% (cooling and solvent reuse)
- waste requiring special treatment
Name: Unreacted phenol, byproducts (phenol derivatives), waste solvent
Approximate quantity: 0.2 – 0.3 kg/kg (waste disposal required)
Reference Process and Background
Production process: Phenol is reacted with carbon dioxide at high temperature and pressure to produce p-hydroxybenzoic acid. If copper oxide is used as a catalyst, it must be regenerated. Reuse of solvents such as cooling water and toluene improves resource efficiency.
Environmental management: Collection of unreacted phenol and disposal of waste are important.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 1.8 – 2.5 kWh/kg |
| Natural gas consumption | 0.03 – 0.05 kg/kg |
| CO₂ emissions other than combustion | 0.05 – 0.08 kg CO₂/kg |
| Water usage | 3 – 5 kg/kg |
| Phenol usage | 0.6 – 0.7 kg/kg |
| carbon dioxide usage | 0.2 – 0.3 kg/kg |
| Catalyst usage | 0.01 – 0.02 kg/kg |
| Catalyst degradation rate | 10 – 15%/kg |
| Solvent usage | 2 – 4 kg/kg |
| Waste (unreacted materials, byproducts) | 0.2 – 0.3 kg/kg |
Carbon monoxide (CO)
Industrially, aqueous gas shift reactions and partial oxidation of methane are used. This section focuses on the aqueous gas shift method (a process that also involves CO₂ and hydrogen gas production).
Input power (kWh/kg)
0.8 – 1.2 kWh/kg
(agitation, temperature control, pressure control)
- input fuel
Type: Natural gas
Approximate quantity:
Natural gas: 0.03 – 0.05 kg/kg (heating for partial oxidation)
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.2 – 0.4 kg CO₂/kg
(from aqueous gas shift reaction and by-products)
- amount of change in resources such as soil, stone, and water (kg)
Water: 1.5 – 2.0 kg/kg (used as steam)
- input item name and input amount guideline
Hydrocarbons (calculated as CH₄): 0.6 – 0.8 kg/kg
Oxygen (O₂): 0.2 – 0.3 kg/kg (for partial oxidation reaction)
Catalyst (nickel catalyst, iron oxide): 0.01 – 0.02 kg/kg
Degradation rate: 10 – 15%/kg (degradation due to continuous use)
- process substances such as solvents
Name: Water
Usage: 1.5 – 2.0 kg/kg (steam supply)
Circulation rate: 70 – 85% (cooling and steam reuse)
- waste requiring special treatment
Name: Byproducts (CO₂), sulfides (upon catalyst degradation)
Approximate quantity: 0.2 – 0.3 kg/kg (proper handling required)
Reference Process and Background
Manufacturing Process:
Aqueous gas shift reaction: CH₄ and H₂O react under high temperature and pressure using a catalyst to produce CO and H₂. CO₂ is emitted as a byproduct. When oxygen is used, CO production is increased by partial oxidation.
Environmental management: The use of steam circulation and CO₂ emission control are important.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.8 – 1.2 kWh/kg |
| Natural gas consumption | 0.03 – 0.05 kg/kg |
| CO₂ emissions other than combustion | 0.2 – 0.4 kg CO₂/kg |
| Water usage | 1.5 – 2.0 kg/kg |
| Hydrocarbons (CH₄ equivalent) | 0.6 – 0.8 kg/kg |
| Oxygen usage | 0.2 – 0.3 kg/kg |
| Catalyst usage | 0.01 – 0.02 kg/kg |
| Catalyst degradation rate | 10 – 15%/kg |
| Solvent usage | 1.5 – 2.0 kg/kg |
| Waste (byproducts) | 0.2 – 0.3 kg/kg |
Ruthenium iodide (RuI₃)
Ruthenium iodide is used in catalysts, electrode materials, and electronic components. A common production method is the direct reaction of ruthenium and iodine.
Input power (kWh/kg)
2.0 – 3.5 kWh/kg
(agitation, temperature control, vacuum drying)
- input fuel
Type: Natural gas
Approximate quantity:
Natural gas: 0.05 – 0.08 kg/kg (heating and drying process)
CO₂ emissions other than combustion (kg CO₂/kg)
0.05 – 0.1 kg CO₂/kg
(reaction and process emissions)
- amount of change in resources such as soil, stone, and water (kg)
Water: 2 – 3 kg/kg (cooling and cleaning)
- input item name and input amount guideline
Ruthenium (Ru): 0.5 – 0.7 kg/kg (main raw material)
Iodine (I₂): 0.6 – 0.8 kg/kg (for iodide reaction)
Catalyst (e.g. ruthenium chloride): 0.01 – 0.02 kg/kg
Degradation rate: 10 – 15%/kg (replacement after multiple uses)
- process substances such as solvents
Name: Acetonitrile (or toluene)
Usage: 1 – 2 kg/kg
Recirculation rate: 80 – 90% (solvent reuse)
- waste requiring special treatment
Name: Unreacted iodine, waste solvent, byproducts (residue of iodine compounds)
Approximate quantity: 0.2 – 0.3 kg/kg (proper disposal required)
Reference Process and Background
Manufacturing Process:
Ruthenium and iodine react to produce ruthenium iodide. After the reaction, the product is filtered, the solvent is removed, and the product is dried.
Environmental management: Proper disposal of unreacted iodine and waste solvents is required.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 2.0 – 3.5 kWh/kg |
| Natural gas consumption | 0.05 – 0.08 kg/kg |
| CO₂ emissions other than combustion | 0.05 – 0.1 kg CO₂/kg |
| Water usage | 2 – 3 kg/kg |
| Ruthenium Usage | 0.5 – 0.7 kg/kg |
| Amount of iodine used | 0.6 – 0.8 kg/kg |
| Catalyst usage | 0.01 – 0.02 kg/kg |
| Catalyst degradation rate | 10 – 15%/kg |
| Solvent usage | 1 – 2 kg/kg |
| Waste (unreacted materials, byproducts) | 0.2 – 0.3 kg/kg |
PGM (Platinum Group Metals) concentrate
PGMs are extracted from ores containing primarily **platinum (Pt), palladium (Pd), rhodium (Rh), iridium (Ir), ruthenium (Ru), and osmium (Os)** and involve a complex process from ore mining to flotation to refining.
Inputs and processes required for the production of PGM concentrates
The following figures are approximate energy and resource inputs to obtain 1 kg of PGM concentrate.
Power input (kWh/kg)
30 – 50 kWh/kg (grinding, flotation, dewatering)
Input fuel
Type: Diesel
Quantity: 0.2 – 0.3 kg/kg (drilling and transport)
CO₂ other than combustion (kg/kg)
0.2 – 0.3 kg CO₂/kg
Amount of soil and water altered.
Waste rock and tailings: 100 – 200 kg/kg
Water: 10 – 20 kg/kg (beneficiation and cooling)
input
Crude ore containing PGMs: 100 – 200 kg/kg
Sulfuric acid: 0.5 – 1.0 kg/kg (for acid leaching of concentrates)
Lime: 0.2 – 0.4 kg/kg (for neutralization)
Solvent usage
Water: 10 – 20 kg/kg
Circulation rate: 70 – 85
waste
Tailings: 100 – 200 kg/kg
Acidic wastewater: requires proper neutralization and treatment
Summary: Quantitative Relationships for PGMs
| stage | amount | PGM content |
| crude ore | 100-200 kg/kg concentrate | 0.5 to 10 ppm (in raw ore) |
| concentrate | 1 kg | 50-500 g/t |
| PGM Metals | 0.05 to 0.5 g | Final collection rate: 60-80%. |
Fluorite (fluorite, CaF₂) concentrate
Fluorite is widely used in the chemical industry and aluminum refining as a raw material for fluorine compounds. Fluorite ore grades are typically 30-50% CaF₂, and concentrates are raised to 90-98% CaF₂ concentration.
Inputs and process for obtaining 1 kg of fluorite concentrate
Input power (kWh/kg)
10 – 15 kWh/kg
(grinding, flotation, dewatering facilities)
- input fuel
Type: Diesel fuel
Approximate quantity: 0.1 – 0.2 kg/kg (mining and transportation)
CO₂ emissions other than combustion (kg CO₂/kg)
0.1 – 0.2 kg CO₂/kg
(e.g., reaction by acid treatment)
- amount of soil and water changed (kg)
Tailings and waste rock: 2 – 4 kg/kg (beneficiation waste)
Water: 5 – 10 kg/kg (cooling and beneficiation)
- input item name and input amount guideline
Fluorite ore (CaF₂ 30-50%): 3 – 4 kg/kg
Sulfuric acid: 0.5 – 1.0 kg/kg (for impurity removal)
Lime (CaO): 0.1 – 0.3 kg/kg (for neutralization)
- process substances such as solvents
Name: Water
Usage: 5 – 10 kg/kg
Recirculation rate: 80 – 90% (cooling and cleaning water reuse)
- waste requiring special treatment
Name: Tailings, sulfuric acid waste
Approximate quantity: 2 – 4 kg/kg
- catalyst use and degradation rate
Catalyst: No specific catalyst is used, but lime is used as a neutralizer
Degradation rate: Lime is discarded after a single use.
Reference Process and Background
Ore mining:
Fluorite ore is mined and crushed. The ore contains 30-50% CaF₂.
Beneficiation (flotation):
Remove impurities and increase the concentration of CaF₂ to over 90%. Iron and calcium-based impurities are removed using sulfuric acid and other chemicals.
Neutralization and dehydration:
Acidic effluent is neutralized with lime, and concentrates are dehydrated and dried after flotation.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 10 – 15 kWh/kg |
| Diesel consumption | 0.1 – 0.2 kg/kg |
| CO₂ emissions other than combustion | 0.1 – 0.2 kg CO₂/kg |
| Tailings and waste rock volume | 2 – 4 kg/kg |
| Water usage | 5 – 10 kg/kg |
| Fluorite ore usage | 3 – 4 kg/kg |
| Sulfuric Acid Usage | 0.5 – 1.0 kg/kg |
| Amount of lime used | 0.1 – 0.3 kg/kg |
| Solvent usage | 5 – 10 kg/kg |
| Waste (tailings, liquid waste) | 2 – 4 kg/kg |
Polymer-based flocculants (e.g., polyacrylamide)
Polymer flocculants are widely used in many industries, including water treatment, pulp and paper, and mining. The following data refer to the process used to polymerize acrylamide to obtain polyacrylamide.
Inputs and process for producing 1 kg of polymeric flocculant
Input power (kWh/kg)
3 – 6 kWh/kg
(polymerization process, stirring, drying and filtration)
- input fuel
Type: Heat derived from natural gas or electricity
Approximate quantity:
Natural gas: 0.1 – 0.15 kg/kg (heating and drying process)
CO₂ emissions other than combustion (kg CO₂/kg)
0.05 – 0.08 kg CO₂/kg
(byproduct of chemical reaction)
- amount of change in resources such as soil, stone, and water (kg)
Water: 2 – 3 kg/kg (for processing and cooling)
- input item name and input amount guideline
Acrylic acid (C3H4O2): 0.6 – 0.8 kg/kg
Acrylamide: 0.3 – 0.5 kg/kg
Initiator (e.g., ammonium persulfate): 0.01 – 0.02 kg/kg
Catalyst degradation rate: 10 – 15% (as initiator)
- process substances such as solvents
Name: Water
Usage: 2 – 3 kg/kg
Circulation rate: 80 – 90% (cooling and cleaning water)
- waste requiring special treatment
Name: Unreacted monomers, byproducts, wastewater
Approximate quantity: 0.1 – 0.3 kg/kg
Reference Process and Background
Polymerization reaction:
Acrylic acid and acrylamide are used in the polymerization reaction in the presence of initiators. Water is used as reaction solvent and for cooling.
Drying and filtration:
After polymerization, the product is filtered and dried and finished as a powder or liquid flocculant.
Treatment of wastewater and unreacted monomers:
Unreacted monomers and wastewater are properly treated to reduce environmental impact.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 3 – 6 kWh/kg |
| Natural gas consumption | 0.1 – 0.15 kg/kg |
| CO₂ emissions other than combustion | 0.05 – 0.08 kg CO₂/kg |
| Water usage | 2 – 3 kg/kg |
| Amount of acrylic acid used | 0.6 – 0.8 kg/kg |
| Amount of acrylamide used | 0.3 – 0.5 kg/kg |
| Amount of initiator used | 0.01 – 0.02 kg/kg |
| Catalyst degradation rate | 10 – 15% of |
| Solvent usage | 2 – 3 kg/kg |
| Waste (wastewater, byproducts) | 0.1 – 0.3 kg/kg |
Potassium chloride (KCl)
Potassium chloride is widely used in potash fertilizers and industrial applications and is obtained from potash ores through a beneficiation and purification process. The typical grade of potash ore is equivalent to 10-25% K₂O, and high purity KCl is obtained in the beneficiation process.
Inputs and process to obtain 1 kg of potassium chloride
Input power (kWh/kg)
1.5 – 3.0 kWh/kg
(grinding, flotation, concentration, dewatering facilities)
- input fuel
Type: Diesel or natural gas
Approximate quantity: 0.05 – 0.1 kg/kg (dry and transport)
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.02 – 0.05 kg CO₂/kg
(from chemical reactions and by-products)
- amount of change in resources such as soil, stone, and water (kg)
Waste rock and tailings: 5 – 8 kg/kg
Water: 2 – 4 kg/kg (for cooling and leaching)
- input item name and input amount guideline
Potash ore (10-25% K₂O): 5 – 10 kg/kg (depending on concentrate grade)
Hydrochloric acid (HCl): 0.1 – 0.2 kg/kg (for removal of impurities)
Lime (CaO): 0.05 – 0.1 kg/kg (for neutralization)
- process substances such as solvents
Name: Water
Usage: 2 – 4 kg/kg
Circulation rate: 85 – 95% (cooling and cleaning reuse)
- waste requiring special treatment
Name: Tailings, sodium chloride waste
Approximate quantity: 5 – 8 kg/kg
- catalyst use and degradation rate
Catalyst: Not used specifically, but lime is used as a neutralizer
Degradation rate: Discarded after single use
Reference Process and Background
Ore mining:
Potassium ore (main components: silver carnite and sylvite) is mined, crushed, and concentrated. Potassium ores contain about 10-25% K₂O.
Beneficiation and refining:
KCl is purified by flotation or thermal treatment. Sodium chloride (NaCl) is generated as a byproduct.
Dehydration and drying:
The concentrate obtained by flotation is dried to produce the final product. By-products such as tailings and sodium chloride solution are properly processed.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 1.5 – 3.0 kWh/kg |
| Diesel consumption | 0.05 – 0.1 kg/kg |
| CO₂ emissions other than combustion | 0.02 – 0.05 kg CO₂/kg |
| Amount of waste rock and tailings | 5 – 8 kg/kg |
| Water usage | 2 – 4 kg/kg |
| Amount of potash ore used | 5 – 10 kg/kg |
| Hydrochloric acid usage | 0.1 – 0.2 kg/kg |
| Amount of lime used | 0.05 – 0.1 kg/kg |
| Solvent usage | 2 – 4 kg/kg |
| Waste (tailings, NaCl effluent) | 5 – 8 kg/kg |
Silica (SiO₂)
Input power (kWh/kg)
2.5 – 5.0 kWh/kg
(grinding, refining, drying process)
- input fuel
Type: Natural gas or electricity
Approximate quantity: 0.05 – 0.1 kg/kg
Applications: As a heating source for high temperature processing and drying
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.02 – 0.05 kg CO₂/kg
(as by-product of chemical processing)
- amount of change in resources such as soil, stone, and water (kg)
Waste rock and tailings: 2 – 4 kg/kg (waste after mining and beneficiation)
Water: 2 – 5 kg/kg (for cleaning and cooling)
- input item name and input amount guideline
Quartz sand (SiO₂ 90-99%): 1.2 – 1.5 kg/kg (for high-purity silica refining)
Hydrochloric acid (HCl): 0.1 – 0.2 kg/kg (for removal of impurities such as iron)
Lime (CaO): 0.05 – 0.1 kg/kg (for neutralization)
- process substances such as solvents
Name: Water
Usage: 2 – 5 kg/kg
Circulation rate: 80 – 90% (reuse of cleaning and cooling water)
- waste requiring special treatment
Name: Tailings, acidic waste liquid (hydrochloric acid)
Approximate quantity: 2 – 4 kg/kg
- catalyst use and degradation rate
Catalyst: No specific catalyst is used, but acids and lime are used in the chemical process
Degradation rate: Acid is discarded or reneutralized after single use
Reference Process and Background
Ore mining
Quartz sand ore is mined to obtain high-purity silica. Mined quartz sand contains 90-99% SiO₂.
Beneficiation and washing
Mined quartz sand is crushed, flotation, or acid treated to remove impurities and increase the concentration of SiO₂. Iron and other impurities are removed with hydrochloric acid.
Drying and Finishing
Finally, the beneficiated silica is dried and finished to the particle size required for the intended use.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 2.5 – 5.0 kWh/kg |
| Natural gas consumption | 0.05 – 0.1 kg/kg |
| CO₂ emissions other than combustion | 0.02 – 0.05 kg CO₂/kg |
| Amount of waste rock and tailings | 2 – 4 kg/kg |
| Water usage | 2 – 5 kg/kg |
| Amount of quartz sand used | 1.2 – 1.5 kg/kg |
| Hydrochloric acid usage | 0.1 – 0.2 kg/kg |
| Amount of lime used | 0.05 – 0.1 kg/kg |
| Solvent usage | 2 – 5 kg/kg |
| Waste (tailings, acidic liquid waste) | 2 – 4 kg/kg |
This data can be used as a basis for evaluating resource efficiency and environmental impact in the production of silica. Actual values may vary slightly depending on ore grade and processing facility conditions.
Palladium (Pd) catalyst
Palladium catalysts are widely used mainly in chemical reactions (reduction, hydrogenation, coupling reactions) and exhaust gas purification, and alumina (Al₂O₃) and activated carbon are often used as base materials.
Inputs and process for producing 1 kg of palladium catalyst
Input power (kWh/kg)
15 – 25 kWh/kg
(required for grinding, dispersing, firing, drying, etc.)
- input fuel
Type: Natural gas
Approximate quantity: 0.1 – 0.2 kg/kg
Applications: Drying and firing processes
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.05 – 0.1 kg CO₂/kg
(generated from treatment of salts, by-products)
- amount of change in resources such as soil, stone, and water (kg)
Wastewater and waste water: 3 – 5 kg/kg
Waste rock and tailings (residuals from ore processing): 200 – 500 kg/kg (depending on refining stage)
- input item name and input amount guideline
PGM ore (Pd content 0.01-0.02%): 500 – 1000 kg/kg
Palladium nitrate (Pd(NO₃)₂): 0.1 – 0.2 kg/kg
Alumina (Al₂O₃) or activated carbon: 0.5 – 0.8 kg/kg
Hydrochloric acid (HCl): 0.2 – 0.5 kg/kg (cleaning and refining)
Sulfuric acid (H₂SO₄): 0.1 – 0.2 kg/kg (for removal of impurities)
- process substances such as solvents
Name: Water
Usage: 3 – 5 kg/kg
Circulation rate: 85 – 95% (for cooling and cleaning)
- waste requiring special treatment
Name: Wastewater, acidic wastewater, PGM tailings
Approximate quantity: 3 – 5 kg/kg
- catalyst use and degradation rate
Catalyst degradation rate: 10 – 15%/kg of palladium catalyst (recyclable)
Spent catalysts are often regenerated, with a metal recovery rate of over 90%.
Reference Process and Background
Mining and refining of PGM ore
Palladium is extracted from ores (typically as a byproduct of nickel or copper ores); to obtain 1 kg of Pd requires 500-1000 kg of ore.
Preparation of palladium nitrate and impregnation of substrate
Palladium nitrate solution is impregnated into the base material (alumina or activated carbon) and calcined.
Drying and firing
Palladium impregnated into the base material is sintered at high temperature to produce a stable catalyst.
Disposal of Waste Liquid
Process byproducts and unused chemicals.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 15 – 25 kWh/kg |
| Natural gas consumption | 0.1 – 0.2 kg/kg |
| CO₂ emissions other than combustion | 0.05 – 0.1 kg CO₂/kg |
| Volume of wastewater | 3 – 5 kg/kg |
| tailings | 200 – 500 kg/kg |
| Amount of PGM ore used | 500 – 1000 kg/kg |
| Palladium nitrate usage | 0.1 – 0.2 kg/kg |
| Alumina or activated carbon | 0.5 – 0.8 kg/kg |
| Hydrochloric acid usage | 0.2 – 0.5 kg/kg |
| Sulfuric Acid Usage | 0.1 – 0.2 kg/kg |
| Water usage | 3 – 5 kg/kg |
| Waste (wastewater, tailings) | 3 – 5 kg/kg |
| Catalyst degradation rate | 10 – 15 |
Linear Alkylbenzene (LAB)
Alkylbenzenes are widely used as raw materials for surfactants and detergents, and are produced through an alkylation reaction between benzene and olefins (alkenes).
Inputs and process to obtain 1 kg of alkylbenzene
Input power (kWh/kg)
3 – 5 kWh/kg
(reaction agitation, cooling, separation and filtration facilities)
- input fuel
Type: Natural gas
Approximate quantity: 0.05 – 0.1 kg/kg
Applications: Used in reaction heating and distillation processes
CO₂ emissions other than combustion (kg CO₂/kg)
0.01 – 0.03 kg CO₂/kg
(as by-product of chemical reaction)
- amount of change in resources such as soil, stone, and water (kg)
Effluents and impurities: 0.5 – 1.0 kg/kg
Water: 2 – 3 kg/kg (cooling and reaction)
- input item name and input amount guideline
Benzene (C₆H₆): 0.3 – 0.4 kg/kg
Alpha olefins (C10 – C14): 0.6 – 0.7 kg/kg
Hydrochloric acid (HCl): 0.05 – 0.1 kg/kg (for catalyst cleaning)
- process substances such as solvents
Name: Water
Usage: 2 – 3 kg/kg
Circulation rate: 85 – 95% (cooling and cleaning water reuse)
- waste requiring special treatment
Name: Unreacted olefins, impurities, acidic wastewater
Approximate quantity: 0.5 – 1.0 kg/kg
- catalyst use and degradation rate
Catalyst: Solid acid catalyst (used for alkylation of benzene and olefins)
Degradation rate: 5 – 10%/kg (periodic regeneration required)
Reference Process and Background
Alkylation reaction
Benzene and alpha-olefin are reacted in the presence of a solid acid catalyst to produce alkylbenzenes. Reaction temperatures of 80-120°C are common.
Separation and Purification
Unreacted materials are separated from the generated alkylbenzene and impurities are removed by distillation or filtration.
Regeneration of effluents and catalysts
Catalysts degrade after several uses and are regenerated or discarded. Hydrochloric acid and other chemicals are used for cleaning.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 3 – 5 kWh/kg |
| Natural gas consumption | 0.05 – 0.1 kg/kg |
| CO₂ emissions other than combustion | 0.01 – 0.03 kg CO₂/kg |
| Volume of liquid waste and impurities | 0.5 – 1.0 kg/kg |
| Water usage | 2 – 3 kg/kg |
| Benzene usage | 0.3 – 0.4 kg/kg |
| Amount of alpha olefin used | 0.6 – 0.7 kg/kg |
| Hydrochloric acid usage | 0.05 – 0.1 kg/kg |
| Solvent usage | 2 – 3 kg/kg |
| Catalyst degradation rate | 5 – 10%/kg |
| Waste (unreacted olefin, impurities) | 0.5 – 1.0 kg/kg |
Vinyl acetates (VAc)
Vinyl acetates are widely used in the production of polyvinyl alcohol, adhesives and coatings. Typical industrial production methods use direct esterification by the reaction of ethylene, acetic acid, and oxygen.
Inputs and process to obtain 1 kg of vinyl acetate
Input power (kWh/kg)
3 – 6 kWh/kg
(reaction facilities, cooling, compression, separation processes)
- input fuel
Type: Natural gas
Approximate quantity: 0.05 – 0.1 kg/kg
Applications: Used for reaction heating and distillation processes
CO₂ emissions other than combustion (kg CO₂/kg)
0.02 – 0.05 kg CO₂/kg
(from side reactions and decomposition of raw materials)
- amount of change in resources such as soil, stone, and water (kg)
Wastewater and acidic wastewater: 1.0 – 2.0 kg/kg
Water: 3 – 5 kg/kg (cooling and reaction process)
- input item name and input amount guideline
Ethylene (C₂H₄): 0.4 – 0.5 kg/kg
Acetic acid (CH₃COOH): 0.3 – 0.4 kg/kg
Oxygen (O₂): 0.1 – 0.2 kg/kg
Hydrochloric acid (HCl): 0.05 – 0.1 kg/kg (used to remove impurities)
- process substances such as solvents
Name: Water
Usage: 3 – 5 kg/kg
Recirculation rate: 85 – 90% (cooling and cleaning water reuse)
- waste requiring special treatment
Name: Acidic liquid waste, unreacted acetic acid
Approximate quantity: 1.0 – 2.0 kg/kg
- catalyst use and degradation rate
Catalyst: Palladium-based catalyst (used for reaction acceleration)
Degradation rate: 5 – 10%/kg (renewable)
Reference Process and Background
esterification reaction
Ethylene, acetic acid, and oxygen react in the presence of a palladium-based catalyst to produce vinyl acetates.
Separation and distillation
The unreacted product is separated from the product and distilled to obtain high-purity vinyl acetate.
Catalyst regeneration
Used catalysts are regenerated and reused, but over time they degrade and need to be replaced.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 3 – 6 kWh/kg |
| Natural gas consumption | 0.05 – 0.1 kg/kg |
| CO₂ emissions other than combustion | 0.02 – 0.05 kg CO₂/kg |
| Volume of wastewater and acidic effluent | 1.0 – 2.0 kg/kg |
| Water usage | 3 – 5 kg/kg |
| Ethylene usage | 0.4 – 0.5 kg/kg |
| Acetic Acid Usage | 0.3 – 0.4 kg/kg |
| Oxygen usage | 0.1 – 0.2 kg/kg |
| Hydrochloric acid usage | 0.05 – 0.1 kg/kg |
| Solvent usage | 3 – 5 kg/kg |
| Catalyst degradation rate | 5 – 10% |
| Waste (acidic liquid waste, unreacted materials) | 1.0 – 2.0 kg/kg |
Butadiene (C₄H₆)
Butadiene is mainly used as a raw material in the manufacture of rubber products and plastics and is obtained as a byproduct of the ethylene cracking process. Extraction and dehydrogenation methods are also used.
Inputs and process to obtain 1 kg of butadiene
Input power (kWh/kg)
2 – 4 kWh/kg
(cracking facility, distillation, gas separation process)
- input fuel
Type: Natural gas
Approximate quantity: 0.05 – 0.1 kg/kg
Applications: For heating (cracking and distillation processes)
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.02 – 0.05 kg CO₂/kg
(impurity degradation and process origin)
- amount of change in resources such as soil, stone, and water (kg)
Wastewater and waste water: 1.0 – 2.0 kg/kg
Cooling water consumption: 3 – 5 kg/kg
- input item name and input amount guideline
Naphtha: 2.5 – 3.0 kg/kg (as by-product from naphtha cracking)
Ethylene (C₂H₄): 0.2 – 0.3 kg/kg (as part of cracking process)
Water vapor: 1.0 – 2.0 kg/kg (to accelerate cracking reaction)
- process substances such as solvents
Name: Water
Usage: 3 – 5 kg/kg
Circulation rate: 80 – 90% (cooling and cleaning water reuse)
- waste requiring special treatment
Name: Waste liquid containing acid gas and impurities
Approximate quantity: 1.0 – 2.0 kg/kg
- catalyst use and degradation rate
Catalyst: Iron oxide or aluminum oxide catalyst (if used for dehydrogenation reaction)
Degradation rate: 10 – 15%/kg (regularly renewable)
Reference Process and Background
naphtha cracking method
Butadiene is obtained in the cracking process, in which naphtha is heated to 800-900°C and cracked. Light olefins such as ethylene, propylene, and butadiene are produced in this process.
Distillation and Separation
Butadiene is separated from the gas mixture produced using distillation and extraction methods.
Catalyst use and regeneration
Iron oxide catalysts are used in the dehydrogenation process, and the catalysts are regenerated periodically.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 2 – 4 kWh/kg |
| Natural gas consumption | 0.05 – 0.1 kg/kg |
| CO₂ emissions other than combustion | 0.02 – 0.05 kg CO₂/kg |
| Volume of wastewater and effluent | 1.0 – 2.0 kg/kg |
| Cooling water usage | 3 – 5 kg/kg |
| Naphtha usage | 2.5 – 3.0 kg/kg |
| Ethylene usage | 0.2 – 0.3 kg/kg |
| Steam usage | 1.0 – 2.0 kg/kg |
| Catalyst degradation rate | 10 – 15 |
| Waste (acid gas, impurities) | 1.0 – 2.0 kg/kg |
Copper(II) chloride (CuCl₂)
Copper dichloride is widely used as a redox catalyst, analytical reagent, or as an intermediate in chemical processes.
Inputs and process to obtain 1 kg of CuCl₂
Input power (kWh/kg)
2 – 4 kWh/kg
(grinding, reaction, filtration, drying process)
- input fuel
Type: Natural gas
Approximate quantity: 0.05 – 0.1 kg/kg
Applications: Heating process (drying, evaporation)
CO₂ emissions other than combustion (kg CO₂/kg)
0.01 – 0.03 kg CO₂/kg
(emissions from process reactions)
- amount of change in resources such as soil, stone, and water (kg)
Effluents and waste: 1 – 2 kg/kg
Water consumption: 2 – 4 kg/kg (for cleaning and cooling)
- input item name and input amount guideline
Copper ore (Cu content 1-2%): 50 – 100 kg/kg
Hydrochloric acid (HCl): 0.8 – 1.0 kg/kg
Oxygen (O₂): 0.05 – 0.1 kg/kg (oxidation reaction accelerator)
- process substances such as solvents
Name: Water
Usage: 2 – 4 kg/kg
Recirculation rate: 80 – 90% (reuse for cleaning and cooling)
- waste requiring special treatment
Name: Acidic liquid waste, tailings
Approximate quantity: 1 – 2 kg/kg
- catalyst use and degradation rate
Catalyst: Iron oxide or activated carbon (used in oxidation process)
Degradation rate: 5 – 10%/kg (renewable)
Reference Process and Background
Oxidation and chloride reaction
Copper obtained from copper ores is reacted with oxygen to produce copper oxide. Hydrochloric acid is then used to produce chloride.
Drying and crystallization
The reaction product is evaporated and dried to yield CuCl₂ crystals.
waste treatment
Byproducts, such as tailings and acidic effluents, are disposed of in an appropriate manner.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 2 – 4 kWh/kg |
| Natural gas consumption | 0.05 – 0.1 kg/kg |
| CO₂ emissions other than combustion | 0.01 – 0.03 kg CO₂/kg |
| Volume of liquid waste and tailings | 1 – 2 kg/kg |
| Water usage | 2 – 4 kg/kg |
| Copper ore usage | 50 – 100 kg/kg |
| Hydrochloric acid usage | 0.8 – 1.0 kg/kg |
| Oxygen usage | 0.05 – 0.1 kg/kg |
| Solvent usage | 2 – 4 kg/kg |
| Catalyst degradation rate | 5 – 10% |
| Waste (acidic liquid waste, tailings) | 1 – 2 kg/kg |
Castor seed (caster seed)
Castor seeds are primarily used as a raw material for **castor oil (caster oil)** and are harvested from agricultural processes.
Inputs and process for obtaining 1 kg of castor seed
Input power (kWh/kg)
0.5 – 1.5 kWh/kg
(use of harvesting machines and processing equipment)
- input fuel
Type: Diesel (fuel for agricultural machinery)
Approximate quantity: 0.1 – 0.2 kg/kg
Applications: Machine operation during cultivation, irrigation, and harvesting.
CO₂ emissions other than combustion (kg CO₂/kg)
0.02 – 0.05 kg CO₂/kg
(indirect emissions from fertilizer and pesticide use)
- amount of change in resources such as soil, stone, and water (kg)
Irrigation water use: 500 – 800 kg/kg
Soil input (fertilizer): 0.5 – 1.0 kg/kg (nitrogen and phosphorus fertilizers)
- input item name and input amount guideline
Seed usage (for sowing): 0.01 – 0.02 kg/kg
Fertilizer (nitrogen fertilizer, phosphate fertilizer): 0.5 – 1.0 kg/kg
Pesticides (herbicides, insecticides): 0.01 – 0.05 kg/kg
- process substances such as solvents
Name: Water (for irrigation and cleaning)
Usage: 500 – 800 kg/kg
Circulation rate: 50 – 70% depending on type of irrigation system
- waste requiring special treatment
Name: Castor seed husk, pesticide residue
Approximate quantity: 0.2 – 0.5 kg/kg
- catalyst use and degradation rate
Catalyst: N/A (no catalyst use in castor seed production)
Reference Process and Background
Cultivation and cultivation process
Castor is grown mainly in India and Brazil and is dependent on the rainy season and irrigation system. It takes 4-5 months from sowing to harvest.
Irrigation and Pesticide Use
Water management through irrigation systems is important, and pesticides are used in appropriate quantities to control pests.
Harvesting and sorting
Seeds are machine harvested and shelled for use. Hulls are discarded or used for compost.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.5 – 1.5 kWh/kg |
| Diesel fuel consumption | 0.1 – 0.2 kg/kg |
| CO₂ emissions other than combustion | 0.02 – 0.05 kg CO₂/kg |
| Irrigation Water Use | 500 – 800 kg/kg |
| Fertilizer use | 0.5 – 1.0 kg/kg |
| Pesticide Use | 0.01 – 0.05 kg/kg |
| Seed usage (for sowing) | 0.01 – 0.02 kg/kg |
| Waste (seed husks, pesticide residues) | 0.2 – 0.5 kg/kg |
Isobutylene (C₄H₈)
Isobutylene is widely used as a raw material for polymers and rubber products and as a fuel additive, and is produced by naphtha cracking and MTBE (methyl tertiary butyl ether) cracking methods.
Inputs and process to obtain 1 kg of isobutylene
Input power (kWh/kg)
2 – 3 kWh/kg
(use of reactors, separators, cooling and distillation facilities)
- input fuel
Type: Natural gas
**Quantity
Approximate weight**: 0.1 – 0.15 kg/kg
Applications: Energy source for heating and distillation processes
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.02 – 0.04 kg CO₂/kg
(process-derived byproduct)
- amount of change in resources such as soil, stone, and water (kg)
Cooling water consumption: 4 – 6 kg/kg
Effluent and waste: 0.5 – 1.0 kg/kg
- input item name and input amount guideline
Naphtha: 2.5 – 3.0 kg/kg (raw material for cracking)
MTBE (Methyl Tertiary Butyl Ether): 1.2 – 1.5 kg/kg (for decomposition method)
Water vapor: 1 – 2 kg/kg (to accelerate cracking)
- process substances such as solvents
Name: Water (for cooling and cleaning)
Usage: 4 – 6 kg/kg
Circulation rate: 85 – 90
- waste requiring special treatment
Name: Acidic liquid waste and cracking residue
Approximate quantity: 0.5 – 1.0 kg/kg
- catalyst use and degradation rate
Catalysts: Aluminum oxide catalysts, zeolite catalysts
Degradation rate: 5 – 10%/kg (periodic regeneration required)
Reference Process and Background
naphtha cracking method
Ethylene, propylene, and isobutylene are produced by the cracking process, in which naphtha is heated to 800-900°C to crack.
MTBE decomposition method
A method of obtaining isobutylene by heating and decomposing MTBE. In many cases, recycling of MTBE is involved in fuel additive applications.
Distillation and Separation
Isobutylene is recovered from the gas mixture produced by distillation and compression.
Catalyst use and regeneration
Aluminum oxide or zeolite catalysts are used to accelerate reactions and are renewable, but must be replaced based on degradation rates.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 2 – 3 kWh/kg |
| Natural gas consumption | 0.1 – 0.15 kg/kg |
| CO₂ emissions other than combustion | 0.02 – 0.04 kg CO₂/kg |
| Cooling water usage | 4 – 6 kg/kg |
| Naphtha usage | 2.5 – 3.0 kg/kg |
| MTBE usage | 1.2 – 1.5 kg/kg |
| Steam usage | 1 – 2 kg/kg |
| Catalyst degradation rate | 5 – 10%/kg |
| Waste (acidic effluents and residues) | 0.5 – 1.0 kg/kg |
Phosphoric acid catalyst 1 kg
Phosphate catalysts are widely used in chemical processes (dehydrogenation, esterification, alkylation, etc.) and are regenerated and replaced to ensure sustainable performance.
Inputs and process for obtaining 1 kg of phosphoric acid-based catalyst
Input power (kWh/kg)
1 – 2 kWh/kg
(reaction, mixing, drying, grinding facilities)
- input fuel
Type: Natural gas
Approximate quantity: 0.05 – 0.1 kg/kg
Applications: Drying and heating processes
CO₂ emissions other than combustion (kg CO₂/kg)
0.01 – 0.03 kg CO₂/kg
(from process reaction)
- amount of change in resources such as soil, stone, and water (kg)
Water consumption: 3 – 5 kg/kg
Volume of liquid waste: 1.0 – 2.0 kg/kg
- input item name and input amount guideline
Phosphoric acid (H₃PO₄): 0.6 – 0.8 kg/kg
Aluminum oxide (Al₂O₃): 0.4 – 0.5 kg/kg
Silica (SiO₂): 0.2 – 0.3 kg/kg
- process substances such as solvents
Name: Water (for reaction and washing)
Usage: 3 – 5 kg/kg
Circulation rate: 70 – 85% (cooling and rinse water reuse)
- waste requiring special treatment
Name: Acidic liquid waste, catalyst powder residue
Approximate quantity: 1.0 – 2.0 kg/kg
- catalyst use and degradation rate
Catalyst usage: 0.1 – 0.15 kg (for process reaction)
Catalyst degradation rate: 10 – 15%/kg (renewable)
Reference Process and Background
Production process for phosphoric acid-based catalysts
Phosphoric acid is mixed with aluminum oxide and silica to form a paste, which is then dried and calcined. This process produces a catalyst with a high surface area.
Distillation and cleaning
The catalyst produced is pulverized to remove unwanted components. Part of the cleaning water is circulated.
Catalyst degradation and regeneration
Catalysts can be regenerated after use, but need to be replaced 10-15% of the time due to gradual degradation.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 1 – 2 kWh/kg |
| Natural gas consumption | 0.05 – 0.1 kg/kg |
| CO₂ emissions other than combustion | 0.01 – 0.03 kg CO₂/kg |
| Water usage | 3 – 5 kg/kg |
| Phosphoric acid usage | 0.6 – 0.8 kg/kg |
| Amount of aluminum oxide used | 0.4 – 0.5 kg/kg |
| Silica usage | 0.2 – 0.3 kg/kg |
| Amount of effluent and waste | 1.0 – 2.0 kg/kg |
| Catalyst degradation rate | 10 – 15%/kg |
Phosphoric anhydride (P₂O₅)
Phosphoric anhydride is obtained through the calcination and refining of phosphate ores and is used in a wide range of applications including chemical fertilizers, catalysts, and esterification.
Inputs and process for obtaining 1 kg of anhydrous phosphoric acid
Input power (kWh/kg)
3 – 5 kWh/kg
(firing furnace, dust collector, drying process)
- input fuel
Type: Natural gas or coke
Approximate quantity: 0.2 – 0.3 kg/kg
Purpose: Used for heating firing furnaces
CO₂ emissions other than combustion (kg CO₂/kg)
0.01 – 0.05 kg CO₂/kg
(from chemical reaction and dust treatment)
- amount of change in resources such as soil, stone, and water (kg)
Phosphorus ore usage: 3 – 5 kg/kg (calculated as 30 – 35% phosphorus content)
Cooling water and wastewater volume: 5 – 7 kg/kg
- input item name and input amount guideline
Phosphate ore (phosphorus content 30-35%): 3 – 5 kg/kg
Oxygen (O₂): 0.1 – 0.2 kg/kg (accelerates firing reaction)
Water vapor: 0.5 – 1.0 kg/kg (for reaction acceleration and dust collection)
- process substances such as solvents
Name: Water (for cooling and cleaning)
Usage: 5 – 7 kg/kg
Circulation rate: 80 – 90
- waste requiring special treatment
Name: Phosphate slag, dust waste
Approximate quantity: 1.5 – 2.0 kg/kg
- catalyst use and degradation rate
Catalyst: calcium oxide (CaO)
Usage: 0.05 – 0.1 kg/kg
Degradation rate: 5 – 10% (renewable)
Reference Process and Background
Firing Process
Phosphate ore is calcined in a high-temperature furnace and reacted with oxygen to produce phosphate gas. This is cooled and condensed to obtain anhydrous phosphoric acid.
Distillation and cooling
The phosphoric acid gas produced is distilled to obtain anhydrous phosphoric acid of the required purity.
Catalyst use and regeneration
Catalysts such as calcium oxide (CaO) are used to stabilize byproducts and remove impurities in phosphate ores.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 3 – 5 kWh/kg |
| Natural gas or coke consumption | 0.2 – 0.3 kg/kg |
| CO₂ emissions other than combustion | 0.01 – 0.05 kg CO₂/kg |
| Phosphorus ore usage | 3 – 5 kg/kg |
| Oxygen usage | 0.1 – 0.2 kg/kg |
| Steam usage | 0.5 – 1.0 kg/kg |
| Cooling water usage | 5 – 7 kg/kg |
| Catalyst degradation rate | 5 – 10% |
| Waste (slag and dust) | 1.5 – 2.0 kg/kg |
Bi-Mo oxide catalyst (Bismuth-molybdenum oxide catalyst)
This catalyst is widely used in chemical reactions such as propylene oxidation and acrylonitrile production.
Inputs and process to obtain 1 kg of Bi-Mo oxide catalyst
Input power (kWh/kg)
2 – 4 kWh/kg
(reactor, drying equipment, crushing and sintering facilities)
- input fuel
Type: Natural gas
Approximate quantity: 0.1 – 0.15 kg/kg
Applications: For heating firing furnaces
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.02 – 0.05 kg CO₂/kg
(emissions from chemical reactions and by-products)
- amount of change in resources such as soil, stone, and water (kg)
Water consumption: 5 – 7 kg/kg
Ore usage: 3 – 5 kg/kg (molybdenum ore, bismuth ore)
- input item name and input amount guideline
Molybdenum ore (Mo content 45-50%): 2 – 3 kg/kg
Bismuth ore (Bi content 80-90%): 1 – 2 kg/kg
Aluminum oxide (carrier): 0.5 – 1.0 kg/kg
- process substances such as solvents
Name: Water (for cleaning and cooling)
Usage: 5 – 7 kg/kg
Circulation rate: 80 – 90
- waste requiring special treatment
Name: Catalyst powder residue, acidic wastewater
Approximate quantity: 0.5 – 1.0 kg/kg
- catalyst use and degradation rate
Catalyst usage: 0.1 – 0.15 kg/kg (reaction process)
Catalyst degradation rate: 5 – 10% (renewable)
Reference Process and Background
Procurement and preparation of raw materials
Bismuth and molybdenum ores are crushed and, if necessary, converted to oxides in an oxidation process.
Mixing and Firing Process
Porous oxide catalysts are produced by mixing the catalyst with a support such as aluminum oxide and calcining it at 800-900°C. The catalyst is then used to produce a porous oxide catalyst.
Drying and Finishing
After calcination, the catalyst is dried and pulverized to a specified particle size.
Catalyst regeneration and disposal
Spent catalysts can be regenerated, but need to be replaced 5-10% of the time due to the rate of deterioration.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 2 – 4 kWh/kg |
| Natural gas consumption | 0.1 – 0.15 kg/kg |
| CO₂ emissions other than combustion | 0.02 – 0.05 kg CO₂/kg |
| Molybdenum ore usage | 2 – 3 kg/kg |
| Bismuth ore usage | 1 – 2 kg/kg |
| Aluminum oxide usage | 0.5 – 1.0 kg/kg |
| Water usage | 5 – 7 kg/kg |
| Catalyst degradation rate | 5 – 10% |
| Waste (powder and acidic liquid waste) | 0.5 – 1.0 kg/kg |
Cyanide-containing waste treatment
Due to their toxicity, cyanide wastes are converted to safe compounds using oxidative decomposition or alkaline hydrolysis methods.
Input and process for treatment of 1 kg of cyanide-containing waste
Input power (kWh/kg)
1 – 2 kWh/kg
(Agitation and pumping equipment, reaction facilities)
- input fuel
Type: Natural gas
Approximate quantity: 0.05 – 0.1 kg/kg
Applications: For heating reactions and evaporation
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.01 – 0.02 kg CO₂/kg
(from chemical reaction and residue treatment)
- amount of change in resources such as soil, stone, and water (kg)
Water consumption: 5 – 7 kg/kg (for reaction and cleaning)
- input item name and input amount guideline
Oxidant (sodium hypochlorite NaClO): 0.1 – 0.2 kg/kg
Alkaline agent (sodium hydroxide NaOH): 0.2 – 0.3 kg/kg
Copper sulfate (CuSO₄, oxidation accelerator): 0.05 – 0.1 kg/kg
- process substances such as solvents
Name: Water (for reaction and cooling)
Usage: 5 – 7 kg/kg
Circulation rate: 80 – 90% (partially reused)
- waste requiring special treatment
Name: Neutralization residue, filtration cake
Approximate quantity: 0.5 – 1.0 kg/kg
- catalyst use and degradation rate
Catalyst: copper sulfate (CuSO₄)
Usage: 0.05 – 0.1 kg/kg
Degradation rate: 5 – 10% (periodic regeneration required)
Reference Process and Background
alkali hydrolysis method
Sodium hydroxide is added to cyanide-containing materials to produce a reaction that reduces toxicity.
oxidative degradation method
Sodium hypochlorite is used to oxidize and detoxify cyanide. Copper sulfate is used as an oxidation accelerator.
Disposal of effluents and residues
After treatment, the residue is filtered, neutralized, and disposed of.
Catalyst regeneration
Copper sulfate is reusable, but should be replaced periodically as it deteriorates.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 1 – 2 kWh/kg |
| Natural gas consumption | 0.05 – 0.1 kg/kg |
| CO₂ emissions other than combustion | 0.01 – 0.02 kg CO₂/kg |
| Water usage | 5 – 7 kg/kg |
| Sodium hypochlorite usage | 0.1 – 0.2 kg/kg |
| Sodium hydroxide usage | 0.2 – 0.3 kg/kg |
| Copper Sulfate Usage | 0.05 – 0.1 kg/kg |
| Catalyst degradation rate | 5 – 10% |
| Waste (e.g., neutralization residue) | 0.5 – 1.0 kg/kg |
Zinc oxide (ZnO)
Zinc oxide is widely used in the chemical industry, rubber industry, ceramics, and battery materials. The following assumes a manufacturing process based on common wet or direct methods.
Inputs and process to obtain 1 kg of zinc oxide
Input power (kWh/kg)
1 – 3 kWh/kg
(concentrate grinding, reactors, drying and sintering facilities)
- input fuel
Type: Natural gas or coke
Approximate quantity: 0.15 – 0.3 kg/kg
Applications: Used in firing processes
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.02 – 0.05 kg CO₂/kg
(from process reactions and flue gas treatment)
- amount of change in resources such as soil, stone, and water (kg)
Water consumption: 3 – 5 kg/kg (for reaction and cleaning)
- input item name and input amount guideline
Zinc concentrate (Zn content 50-60%): 1.6 – 2.0 kg/kg
Oxygen (O₂): 0.2 – 0.4 kg/kg (for oxidation reaction)
- process substances such as solvents
Name: Water (cooling and reaction)
Usage: 3 – 5 kg/kg
Circulation rate: 80 – 90
- waste requiring special treatment
Name: Furnace slag, flue gas treatment residue
Approximate quantity: 0.3 – 0.5 kg/kg
- catalyst use and degradation rate
Catalyst: Iron oxide (Fe₂O₃, oxidation accelerator)
Usage: 0.05 – 0.1 kg/kg
Degradation rate: 5 – 8%.
Reference Process and Background
Procurement and preparation of raw materials
Zinc concentrates (containing ZnS) are crushed and calcined in an oxidation furnace to convert them into zinc oxides.
Firing Process
Zinc is oxidized under high temperature conditions (900-1200°C) while oxygen is supplied. Natural gas or coke is used as the heating source.
Exhaust gas treatment and cooling
The byproducts generated by calcination are treated as waste gas and the zinc oxide powder is recovered.
Catalyst regeneration and management
Iron oxide catalysts promote oxidation and are renewable, but with a degradation rate of 5-8%.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 1 – 3 kWh/kg |
| Natural gas or coke consumption | 0.15 – 0.3 kg/kg |
| CO₂ emissions other than combustion | 0.02 – 0.05 kg CO₂/kg |
| Zinc concentrate usage | 1.6 – 2.0 kg/kg |
| Oxygen consumption | 0.2 – 0.4 kg/kg |
| Water usage | 3 – 5 kg/kg |
| Catalyst degradation rate | 5 – 8% |
| Waste (slag and residue) | 0.3 – 0.5 kg/kg |
Acid catalysts (e.g., zeolite-based or sulfuric acid-supported catalysts)
Acid catalysts are widely used as proton-donating catalysts in chemical reactions and play an important role in the petrochemical and polymer industries.
Inputs and process to obtain 1 kg of acid catalyst
Input power (kWh/kg)
3 – 5 kWh/kg
(grinding, drying, calcination, filtration of raw materials)
- input fuel
Type: Natural gas or coke
Approximate quantity: 0.1 – 0.15 kg/kg
Applications: Used in calcination and activation processes
CO₂ emissions other than combustion (kg CO₂/kg)
0.01 – 0.03 kg CO₂/kg
(emissions from by-products during reaction and refining)
- amount of change in resources such as soil, stone, and water (kg)
Water consumption: 5 – 7 kg/kg (for cleaning and cooling)
Ore input: 2 – 3 kg/kg (silica concentrate or alumina)
- input item name and input amount guideline
Silica concentrate (SiO₂ content 95%): 1.5 – 2.0 kg/kg
Alumina (Al₂O₃ content 98%): 0.5 – 1.0 kg/kg
Sulfuric acid (H₂SO₄): 0.2 – 0.3 kg/kg (for acid loading)
- process substances such as solvents
Name: Water (for cleaning and cooling)
Usage: 5 – 7 kg/kg
Circulation rate: 80 – 90
- waste requiring special treatment
Name: Cleaning waste liquid, calcination residue
Approximate quantity: 0.3 – 0.6 kg/kg
- catalyst use and degradation rate
Catalyst: Acid-supported materials (e.g., sulfuric acid-supported alumina)
Usage: 0.1 – 0.15 kg/kg (process dependent)
Degradation rate: 5 – 10% (can be regenerated, but must be replaced as it degrades)
Reference Process and Background
Procurement and mixing of raw materials
Acid zeolite and alumina are prepared from silica and alumina concentrates and acidified with sulfuric acid.
Firing and activation
Fired at high temperatures (500-700°C) to produce an active surface.
Cleaning and Finishing
After washing, it is dried to obtain the final acidic catalyst.
Catalyst regeneration and management
Catalysts can be regenerated after use, but will deteriorate by 5-10%.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 3 – 5 kWh/kg |
| Natural gas or coke consumption | 0.1 – 0.15 kg/kg |
| CO₂ emissions other than combustion | 0.01 – 0.03 kg CO₂/kg |
| Silica concentrate usage | 1.5 – 2.0 kg/kg |
| Alumina usage | 0.5 – 1.0 kg/kg |
| Sulfuric Acid Usage | 0.2 – 0.3 kg/kg |
| Water usage | 5 – 7 kg/kg |
| Catalyst degradation rate | 5 – 10% |
Potassium sulfate (K₂SO₄)
Potassium sulfate is a compound used primarily in fertilizer and industrial applications, either by the Mannheim method or by the ion exchange method. Here, we refer to the typical Mannheim method.
Inputs and process to obtain 1 kg of potassium sulfate
Input power (kWh/kg)
1 – 2 kWh/kg
(operation of agitator, pump, and dryer)
- input fuel
Type: Natural gas or coal
Approximate quantity: 0.2 – 0.3 kg/kg
Applications: Heating process for reaction with sulfuric acid
CO₂ emissions other than combustion (kg CO₂/kg)
0.01 – 0.02 kg CO₂/kg
(emissions from impurities generated during the reaction)
- amount of change in resources such as soil, stone, and water (kg)
Water consumption: 2 – 3 kg/kg (for cooling and cleaning)
**Potassium salt concentrate (containing KCl)
Approximate inputs and energy for the production of 1 kg of potassium sulfate
Input power (kWh/kg)
1 – 2 kWh/kg
(Agitation, pumps, drying equipment in operation)
- input fuel
Type: Natural gas or coal
Approximate quantity: 0.2 – 0.3 kg/kg
(for heating purpose of reaction acceleration)
CO₂ emissions other than combustion (kg CO₂/kg)
0.01 – 0.02 kg CO₂/kg
(Emissions from impurities and side reactions)
- amount of change in resources such as soil, stone, and water (kg)
Water consumption: 2 – 3 kg/kg (reaction cooling and washing)
- input item name and input amount guideline
Potassium salt concentrate (KCl, 90-95% content): 1.3 – 1.5 kg/kg
Sulfuric acid (H₂SO₄): 0.7 – 0.8 kg/kg
(Reaction of sulfuric acid with KCl to form potassium sulfate)
- process substances such as solvents
Name: Water (for cooling and cleaning)
Usage: 2 – 3 kg/kg
Circulation rate: 80 – 90% (partially reused)
- waste requiring special treatment
Name: By-products (salt impurities, filtration residue)
Approximate quantity: 0.1 – 0.3 kg/kg
- catalyst use and degradation rate
Catalyst: not used (Mannheim method does not require a catalyst)
Reference Process and Background
Mannheim process
Potassium chloride (KCl) and sulfuric acid (H₂SO₄) react at high temperature to produce potassium sulfate (K₂SO₄). Hydrogen chloride (HCl) is generated as a byproduct, which requires gas treatment.
Heating and Drying
Because the reaction requires high temperatures (about 600°C), natural gas or coal is generally used for heating.
Waste Disposal
Impurities and filtration residues from the reaction are discarded, but some are reused.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 1 – 2 kWh/kg |
| Natural gas or coal consumption | 0.2 – 0.3 kg/kg |
| CO₂ emissions other than combustion | 0.01 – 0.02 kg CO₂/kg |
| KCl concentrate usage | 1.3 – 1.5 kg/kg |
| Sulfuric Acid Usage | 0.7 – 0.8 kg/kg |
| Water usage | 2 – 3 kg/kg |
| Catalyst degradation rate | na |
| Waste (salts and residues) | 0.1 – 0.3 kg/kg |
Cobalt oxide (Co₃O₄)
Input power (kWh/kg)
2 – 4 kWh/kg
(Used for grinding, stirring, drying and baking processes)
- input fuel
Type: Natural gas or coke
Approximate quantity: 0.1 – 0.2 kg/kg
(for heating in the baking process)
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.02 – 0.05 kg CO₂/kg
(Emissions due to impurities and byproducts)
- amount of change in resources such as soil, stone, and water (kg)
Water consumption: 1 – 3 kg/kg (for cooling and cleaning)
Cobalt concentrate used: 3 – 5 kg/kg (10-40% content)
- input item name and input amount guideline
Cobalt concentrate (Co content 30%): 3.3 – 4.0 kg/kg
Oxygen (O₂): 0.2 – 0.3 kg/kg (used for oxidation reaction)
- process substances such as solvents
Name: Water (for cleaning and cooling)
Usage: 1 – 3 kg/kg
Circulation rate: 80 – 90
- waste requiring special treatment
Name: Furnace slag, filtration residue
Approximate quantity: 0.2 – 0.5 kg/kg
- catalyst use and degradation rate
Catalyst: Catalyst for accelerated oxidation (e.g., iron oxide)
Usage: 0.05 – 0.1 kg/kg
Degradation rate: 5 – 8% (renewable)
Reference Process and Background
Firing Process
Cobalt concentrate is calcined in an oxidation furnace to produce cobalt oxide (Co₃O₄). Oxidation is accelerated at high temperatures while oxygen is supplied.
Heating and Oxidation
Firing temperatures of 500-800°C are required, and natural gas or coke is used as fuel.
Cooling and cleaning
After calcination, the cobalt oxide is cooled, cleaned, and powdered as the final product.
Catalysts
Catalysts for accelerated oxidation (e.g., iron oxide) can be regenerated, but will degrade by 5-8% per kg of production.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 2 – 4 kWh/kg |
| Natural gas or coke consumption | 0.1 – 0.2 kg/kg |
| CO₂ emissions other than combustion | 0.02 – 0.05 kg CO₂/kg |
| Cobalt concentrate usage | 3.3 – 4.0 kg/kg |
| Oxygen consumption | 0.2 – 0.3 kg/kg |
| Water usage | 1 – 3 kg/kg |
| Catalyst degradation rate | 5 – 8% |
| Waste (slag and residue) | 0.2 – 0.5 kg/kg |
Vinyl acetate monomer (VAM)
Input power (kWh/kg)
2 – 3 kWh/kg
(Operation of pumps, agitators, and reaction controllers)
- input fuel
Type: Natural gas
Approximate quantity: 0.2 – 0.3 kg/kg
(heating and distillation in the reactor)
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.05 – 0.08 kg CO₂/kg
(emissions from side reactions and decomposition of raw materials)
- amount of change in resources such as soil, stone, and water (kg)
Water consumption: 2 – 4 kg/kg (for cooling and solvent regeneration)
Oxygen consumption: 0.6 – 0.8 kg/kg (ethylene oxidation reaction)
- input item name and input amount guideline
Ethylene: 0.5 – 0.6 kg/kg
Acetic acid: 0.5 – 0.6 kg/kg
(Acetic acid reacts with ethylene to produce VAM)
Oxygen: 0.6 – 0.8 kg/kg
(Used in oxidative reactions)
- process substances such as solvents
Name: Water (for cooling and cleaning)
Usage: 2 – 4 kg/kg
Circulation rate: 80 – 90
- waste requiring special treatment
Name: Reaction residue, filtered impurities
Approximate quantity: 0.1 – 0.2 kg/kg
- catalyst use and degradation rate
Catalyst: Palladium-based catalyst
Usage: 0.01 – 0.02 kg/kg
Degradation rate: 5 – 10% (renewable, but degrades with each use)
Reference Process and Background
Oxidation reaction of ethylene and acetic acid
Acetic acid and ethylene react in the presence of oxygen to produce VAM. Palladium catalysts are often used in this process.
Distillation and Purification
The generated VAM is distilled to remove impurities. Natural gas is used as a heating source to control the temperature in the reactor.
Catalyst management
Palladium-based catalysts can be regenerated, but need to be replaced about 5-10% of the time due to degradation that occurs.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 2 – 3 kWh/kg |
| Natural gas consumption | 0.2 – 0.3 kg/kg |
| CO₂ emissions other than combustion | 0.05 – 0.08 kg CO₂/kg |
| Ethylene usage | 0.5 – 0.6 kg/kg |
| Acetic Acid Usage | 0.5 – 0.6 kg/kg |
| Oxygen consumption | 0.6 – 0.8 kg/kg |
| Water usage | 2 – 4 kg/kg |
| Catalyst degradation rate | 5 – 10% |
| Waste (residues, impurities) | 0.1 – 0.2 kg/kg |
Benzoic acid
Input power (kWh/kg)
2 – 4 kWh/kg
(stirring, pumping, cooling and distillation operations)
- input fuel
Type: Natural gas or heavy oil
Approximate quantity: 0.2 – 0.3 kg/kg
(for reactor heating and distillation)
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.05 – 0.1 kg CO₂/kg
(Emissions from decomposition of reaction by-products)
- amount of change in resources such as soil, stone, and water (kg)
Water consumption: 2 – 3 kg/kg (cooling and washing)
- input item name and input amount guideline
Toluene: 0.9 – 1.1 kg/kg
(Raw material for oxidation)
Oxygen: 0.4 – 0.5 kg/kg
(Used in the oxidation reaction of toluene)
- process substances such as solvents
Name: Water
Usage: 2 – 3 kg/kg (for cooling and washing after reaction)
Circulation rate: 80 – 90
- waste requiring special treatment
Name: Side reaction products (organic residues, unreacted toluene)
Approximate quantity: 0.1 – 0.2 kg/kg
- catalyst use and degradation rate
Catalyst: Cobalt or manganese-based oxidation catalyst
Usage: 0.01 – 0.03 kg/kg
Degradation rate: 5 – 10% (renewable)
Reference Process and Background
Toluene oxidation method
The industrial production of benzoic acid is primarily accomplished by the process of oxidizing toluene with oxygen. Cobalt or manganese-based catalysts are used to promote oxidation.
Reaction conditions
Oxidation takes place under high temperature (150-200°C) and pressure conditions, producing water and unreacted toluene as byproducts.
Catalyst regeneration
Oxidation catalysts are renewable, with a degradation rate of 5-10%.
Distillation and Purification
After the reaction, the benzoic acid is distilled to remove impurities.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 2 – 4 kWh/kg |
| Natural gas or fuel oil consumption | 0.2 – 0.3 kg/kg |
| CO₂ emissions other than combustion | 0.05 – 0.1 kg CO₂/kg |
| Toluene usage | 0.9 – 1.1 kg/kg |
| Oxygen consumption | 0.4 – 0.5 kg/kg |
| Water usage | 2 – 3 kg/kg |
| Catalyst degradation rate | 5 – 10% |
| Waste (e.g., organic residues) | 0.1 – 0.2 kg/kg |
Iron chloride (FeCl₃)
Input power (kWh/kg)
1 – 2 kWh/kg
(pumps, agitators, distillation and cooling processes)
- input fuel
Type: Natural gas or heavy oil
Approximate quantity: 0.1 – 0.2 kg/kg
(for reaction heating and drying)
CO₂ emissions other than combustion (kg CO₂/kg)
0.02 – 0.05 kg CO₂/kg
(emissions from chemical reactions and by-products)
- amount of change in resources such as soil, stone, and water (kg)
Water consumption: 1 – 3 kg/kg (for cooling and dissolution)
- input item name and input amount guideline
Iron ore concentrate (Fe content 50%): 1.6 – 2.0 kg/kg
Hydrochloric acid (HCl): 0.6 – 0.8 kg/kg
- process substances such as solvents
Name: Water (for cooling and dissolving)
Usage: 1 – 3 kg/kg
Circulation rate: 85 – 90
- waste requiring special treatment
Name: Unreacted minerals, slag, acidic by-products
Approximate quantity: 0.1 – 0.3 kg/kg
- catalyst use and degradation rate
Catalyst: Iron oxide (used as an oxidation aid in the process)
Usage: 0.01 – 0.02 kg/kg
Degradation rate: 5 – 7% (renewable)
Reference Process and Background
Reaction of iron ore with hydrochloric acid
Iron chloride is produced by reacting iron ore concentrate with hydrochloric acid. Oxides are produced by side reactions and must be removed.
Heating and Drying
The resulting iron chloride solution is concentrated and dried to a solid or crystallized form. Natural gas or heavy oil is mainly used in this process.
Catalyst management
Iron oxide can be reused as a catalyst, with a degradation rate of 5-7%.
Waste Disposal
Acidic effluents and unreacted minerals that remain as byproducts require appropriate treatment.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 1 – 2 kWh/kg |
| Natural gas or fuel oil consumption | 0.1 – 0.2 kg/kg |
| CO₂ emissions other than combustion | 0.02 – 0.05 kg CO₂/kg |
| Iron Ore Concentrate Usage | 1.6 – 2.0 kg/kg |
| Hydrochloric acid usage | 0.6 – 0.8 kg/kg |
| Water usage | 1 – 3 kg/kg |
| Catalyst degradation rate | 5 – 7% |
| Waste (slag, by-products) | 0.1 – 0.3 kg/kg |
Dichloromethane (CH₂Cl₂)
Input power (kWh/kg)
2 – 4 kWh/kg
(Stirring, cooling and refining processes)
- input fuel
Type: Natural gas
Approximate quantity: 0.2 – 0.3 kg/kg
(Reaction heating and distillation process)
CO₂ emissions other than combustion (kg CO₂/kg)
0.03 – 0.06 kg CO₂/kg
(Chlorination reaction by-products and impurity decomposition)
- amount of change in resources such as soil, stone, and water (kg)
Water consumption: 3 – 5 kg/kg (cooling and refining processes)
- input item name and input amount guideline
Methane: 0.3 – 0.4 kg/kg
(raw material for chlorination reaction)
Chlorine (Cl₂): 0.9 – 1.1 kg/kg
(Chlorination reaction with methane)
- process substances such as solvents
Name: Water (cooling, cleaning)
Usage: 3 – 5 kg/kg
Circulation rate: 85 – 90
- waste requiring special treatment
Name: Chlorinated by-products (chloroform, etc.)
Approximate quantity: 0.1 – 0.2 kg/kg
- catalyst use and degradation rate
Catalyst: Iron catalyst or copper catalyst (for chlorination promotion)
Usage: 0.01 – 0.02 kg/kg
Degradation rate: 5 – 10% (renewable)
Reference Process and Background
Chlorination reaction of methane
The chlorination reaction of methane and chlorine produces halides including dichloromethane. Since the product contains chloroform and other substances, separation and purification are necessary.
Reaction Conditions and Purification
The reaction takes place at approximately 300-400°C, and the products are separated and purified by distillation.
Catalyst management
Iron- or copper-based catalysts can be regenerated, but will degrade by 5-10% with each use.
Waste Management
Chloroform and hydrochloric acid are generated as byproducts, which must be properly collected and treated.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 2 – 4 kWh/kg |
| Natural gas consumption | 0.2 – 0.3 kg/kg |
| CO₂ emissions other than combustion | 0.03 – 0.06 kg CO₂/kg |
| Methane usage | 0.3 – 0.4 kg/kg |
| Chlorine usage | 0.9 – 1.1 kg/kg |
| Water usage | 3 – 5 kg/kg |
| Catalyst degradation rate | 5 – 10% |
| Waste (chlorinated by-products) | 0.1 – 0.2 kg/kg |
chlorinated gas treatment
Input power (kWh/kg)
1.5 – 2.5 kWh/kg
(Used for pumps, agitators and washing tower operations)
- input fuel
Type: Natural gas
Approximate quantity: 0.1 – 0.15 kg/kg
(Used for gas heating and absorption tower operations)
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.02 – 0.05 kg CO₂/kg
(Emissions from chemical reactions of by-products)
- amount of change in resources such as soil, stone, and water (kg)
Water consumption: 5 – 8 kg/kg (for cleaning and cooling)
- input item name and input amount guideline
Sodium hydroxide (NaOH): 0.6 – 1.0 kg/kg
(for neutralization and absorption of chlorine gas)
Sulfuric acid (H₂SO₄): 0.2 – 0.3 kg/kg
(pH adjustment and regeneration process)
- process substances such as solvents
Name: Water
Usage: 5 – 8 kg/kg
Circulation rate: 80 – 90
- waste requiring special treatment
Name: Sodium chloride (NaCl), other salts
Approximate quantity: 0.8 – 1.2 kg/kg
(Byproduct of neutralization reaction)
- catalyst use and degradation rate
Catalyst: Activated carbon (for chlorine adsorption)
Usage: 0.05 – 0.1 kg/kg
Degradation rate: 5 – 8%.
Reference Process and Background
Neutralization and absorption treatment of chlorine gas
Chlorine gas is usually neutralized with an alkaline solution (NaOH) and absorbed as chloride. In some cases, activated carbon or adsorbents are used.
Reclamation and Byproduct Management
Sodium chloride produced from the absorbed gas is a byproduct and is properly disposed of.
Catalyst Management
As the adsorption capacity of activated carbon deteriorates, 5-8% of the amount used is replaced.
Improved energy efficiency
Process efficiency is improved through gas cooling and the use of circulating water in absorption towers.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 1.5 – 2.5 kWh/kg |
| Natural gas consumption | 0.1 – 0.15 kg/kg |
| CO₂ emissions other than combustion | 0.02 – 0.05 kg CO₂/kg |
| Sodium hydroxide usage | 0.6 – 1.0 kg/kg |
| Sulfuric Acid Usage | 0.2 – 0.3 kg/kg |
| Water usage | 5 – 8 kg/kg |
| Catalyst degradation rate | 5 – 8% |
| Waste (salts) | 0.8 – 1.2 kg/kg |
Calcium carbonate (CaCO₃)
Input power (kWh/kg)
0.2 – 0.5 kWh/kg
(for grinding, stirring, and drying processes)
- input fuel
Type: Natural gas
Approximate quantity: 0.05 – 0.1 kg/kg
(for drying and baking processes)
CO₂ emissions other than combustion (kg CO₂/kg)
0.05 – 0.1 kg CO₂/kg
(Byproduct of the decomposition reaction of carbonates in raw materials)
- amount of change in resources such as soil, stone, and water (kg)
Water consumption: 0.5 – 1.0 kg/kg
(Used in crushing and cleaning processes)
- input item name and input amount guideline
Limestone concentrate (CaCO₃ ore): 1.5 – 2.0 kg/kg
(dependent on CaCO₃ content)
Carbon dioxide (CO₂): 0.1 – 0.15 kg/kg
(Synthesis method uses CO₂ gas)
- process substances such as solvents
Name: Water
Usage: 0.5 – 1.0 kg/kg
Circulation rate: 80 – 90
- waste requiring special treatment
Name: Sludge (insoluble impurities)
Approximate quantity: 0.05 – 0.1 kg/kg
- catalyst use and degradation rate
Catalyst: None (catalysts are not normally required for calcium carbonate production)
Reference Process and Background
Difference between synthetic and naturally occurring methods
Calcium carbonate is produced either by grinding and drying natural limestone or by synthesizing calcium hydroxide by blowing CO₂ into it. The synthetic method yields a higher purity CaCO₃.
Energy efficiency and drying process
Grinding and drying use electricity and fuel, but the drying process is particularly energy-intensive.
Water recycling
Water is used in the crushing and washing processes, reducing consumption through recycling.
waste treatment
Sludge containing impurities is generated during the manufacturing process and requires appropriate treatment.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.2 – 0.5 kWh/kg |
| Natural gas consumption | 0.05 – 0.1 kg/kg |
| CO₂ emissions other than combustion | 0.05 – 0.1 kg CO₂/kg |
| Limestone concentrate usage | 1.5 – 2.0 kg/kg |
| carbon dioxide usage | 0.1 – 0.15 kg/kg |
| Water usage | 0.5 – 1.0 kg/kg |
| Waste (sludge) | 0.05 – 0.1 kg/kg |
Molybdenum concentrate (MoS₂)
Input power (kWh/kg)
1.5 – 3.0 kWh/kg
(for crushing, beneficiation, flotation beneficiation, etc.)
- input fuel
Type: Light oil (for operating beneficiation equipment)
Approximate quantity: 0.05 – 0.1 kg/kg
(fuel for ore hauling trucks and generators)
CO₂ emissions other than combustion (kg CO₂/kg)
0.01 – 0.05 kg CO₂/kg
(emissions from chemical processing and water use)
- amount of change in resources such as soil, stone, and water (kg)
Water consumption: 5 – 8 kg/kg
(used in flotation and grinding processes)
Waste rock emissions: 3 – 5 kg/kg
(Waste rock remaining after beneficiation)
- input item name and input amount guideline
Raw ore (Mo content 0.1-0.5%): 200 – 300 kg/kg
(ore volume considering molybdenum content)
Lime (for pH adjustment): 0.2 – 0.5 kg/kg
(pH control of flotation process)
- process substances such as solvents
Name: Agent (reagent for flotation: xylene, alkyl ether)
Usage: 0.01 – 0.05 kg/kg
Circulation rate: 85 – 90
- waste requiring special treatment
Name: Flotation waste liquid, tailings
Approximate quantity: 5 – 7 kg/kg
- catalyst use and degradation rate
Catalyst: None (catalysts are generally not used for beneficiation)
Reference Process and Background
Ore mining and beneficiation
Due to the low content of molybdenum ore (MoS₂), a large amount of raw ore is required. The concentrate is concentrated using the flotation beneficiation method.
Use of flotation reagents
Flotation reagents such as alkyl ethers are used to separate molybdenum. Water and lime are used to adjust pH and increase beneficiation efficiency.
Waste and Effluent Management
Tailings after flotation must be properly managed and water recycling is required.
Conclusion.
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 1.5 – 3.0 kWh/kg |
| Fuel consumption | 0.05 – 0.1 kg/kg |
| CO₂ emissions other than combustion | 0.01 – 0.05 kg CO₂/kg |
| Raw ore usage | 200 – 300 kg/kg |
| Lime usage | 0.2 – 0.5 kg/kg |
| Water consumption | 5 – 8 kg/kg |
| Waste (tailings) | 5 – 7 kg/kg |
cobalt (Co)
Input power (kWh/kg)
35 – 45 kWh/kg
(Used in electrolytic refining and refining processes)
- input fuel
Type: Light oil, coal (heat treatment and melting process)
Approximate quantity: 0.05 – 0.15 kg/kg
CO₂ emissions other than combustion (kg CO₂/kg)
0.1 – 0.2 kg CO₂/kg
(emissions from process water and chemical treatment)
- amount of change in resources such as soil, stone, and water (kg)
Water consumption: 10 – 15 kg/kg
(electrolyte or cooling water)
Waste and slag emissions: 5 – 8 kg/kg
(Slag after metallurgical treatment)
- input item name and input amount guideline
Cobalt concentrate (Co content 10-30%): 3 – 5 kg/kg
(raw material before electrolysis and reduction processes)
Sulfuric acid: 0.5 – 1.0 kg/kg
(Used as electrolyte)
- process substances such as solvents
Name: Extraction solvent (organic solvent, acid)
Usage: 0.05 – 0.1 kg/kg
Circulation rate: 90 – 95
- waste requiring special treatment
Name: Electrolysis sludge, tailings, liquid waste
Approximate quantity: 0.5 – 1.5 kg/kg
- catalyst use and degradation rate
Catalyst: None (no catalyst is used in electrolytic refining)
Reference Process and Background
Production of cobalt metal from cobalt concentrate
Cobalt is mainly extracted from sulfide ores and refined by a hydrometallurgical process using a sulfuric acid solution.
Use of electrolysis
Electrolytic refining yields high-purity cobalt and relatively high electricity consumption.
Chemicals and extraction solvents
Many of the organic solvents and acids used in the solvent extraction process are reused.
summary
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 35 – 45 kWh/kg |
| Fuel consumption | 0.05 – 0.15 kg/kg |
| CO₂ emissions other than combustion | 0.1 – 0.2 kg CO₂/kg |
| Concentrate usage | 3 – 5 kg/kg |
| Sulfuric Acid Usage | 0.5 – 1.0 kg/kg |
| Water consumption | 10 – 15 kg/kg |
| Waste and slag | 5 – 8 kg/kg |
barium carbonate (BaCO3)
Input power (kWh/kg)
2 – 4 kWh/kg
(Refining and firing process)
- input fuel
Type: Natural gas or coal
Approximate quantity: 0.05 – 0.08 kg/kg
(Used in the baking process)
CO₂ emissions other than combustion (kg CO₂/kg)
0.2 – 0.3 kg CO₂/kg
(from process chemical reactions and by-products)
- amount of change in resources such as soil, stone, and water (kg)
Water consumption: 1.5 – 2.0 kg/kg
(Cleaning and reaction application)
By-products: barium slag: 0.5 – 0.8 kg/kg
- input item name and input amount guideline
Barium sulfate concentrate (BaSO₄): 1.5 – 2.0 kg/kg
(based on main raw materials and elemental content)
Sodium carbonate (Na₂CO₃): 0.3 – 0.5 kg/kg
(Used for barium conversion reaction)
- process substances such as solvents
Name: Sodium hydroxide solution
Usage: 0.1 – 0.2 kg/kg
Circulation rate: 85 – 90
- waste requiring special treatment
Name: Barium slag, waste liquid
Approximate quantity: 0.5 – 0.8 kg/kg
- catalyst use and degradation rate
Catalyst: None (process does not require a catalyst)
Process Overview and Background
Process of barium carbonate production
Barium carbonate is produced mainly from barium sulfate ore by reaction with sodium carbonate.
The use of fuel is essential because the firing process consumes a lot of energy.
Water and byproduct treatment
Water is required to clean the product, and proper treatment of the byproduct, barium slag, is required.
summary
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 2 – 4 kWh/kg |
| Fuel consumption | 0.05 – 0.08 kg/kg |
| CO₂ emissions other than combustion | 0.2 – 0.3 kg CO₂/kg |
| Barium Sulfate Concentrate Usage | 1.5 – 2.0 kg/kg |
| Sodium carbonate usage | 0.3 – 0.5 kg/kg |
| Water consumption | 1.5 – 2.0 kg/kg |
| Waste volume (slag, etc.) | 0.5 – 0.8 kg/kg |
TiCl₄ (titanium tetrachloride)
Input power (kWh/kg)
2 – 4 kWh/kg
(control of milling, mixing, and reaction processes)
- input fuel
Type: Coke or natural gas
Approximate quantity: 0.4 – 0.6 kg/kg
(to facilitate high-temperature chlorination reactions)
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.1 – 0.15 kg CO₂/kg
(generated from reaction byproducts)
- amount of change in resources such as soil, stone, and water (kg)
Ilmenite concentrate used: 2.0 – 2.5 kg/kg
(Used as a source of TiO₂)
- input item name and input amount guideline
Ilmenite concentrate (FeTiO₃): 2.0 – 2.5 kg/kg
Chlorine (Cl₂): 1.5 – 2.0 kg/kg
(Used in chlorination reaction to produce titanium tetrachloride)
- process substances such as solvents
Name: Chlorine (Cl₂)
Usage: 1.5 – 2.0 kg/kg
Circulation rate: 80 – 90
(recovered and reused after the reaction)
- waste requiring special treatment
Name: Iron chloride (FeCl₃), waste hydrochloric acid
Approximate quantity: 0.5 – 0.8 kg/kg
(Produced from the iron in ilmenite)
- catalyst use and degradation rate
Catalyst: None (no catalyst required for chlorination process at high temperature)
Process Overview and Background
Production process: ilmenite (FeTiO₃) is used as the main raw material, and titanium tetrachloride (TiCl₄) is produced by chlorination reaction. Iron chloride (FeCl₃) is produced as a byproduct, so disposal and reuse of byproducts are important.
Energy consumption: Large amounts of energy are required for high-temperature reactions, and natural gas or coke is used as fuel.
Environmental impact management: Recovery and reuse of chlorine and appropriate treatment of waste hydrochloric acid contribute to improved process efficiency and reduced environmental impact.
summary
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 2 – 4 kWh/kg |
| Fuel consumption | 0.4 – 0.6 kg/kg |
| CO₂ emissions other than combustion | 0.1 – 0.15 kg CO₂/kg |
| Ilmenite concentrate usage | 2.0 – 2.5 kg/kg |
| Chlorine usage | 1.5 – 2.0 kg/kg |
| Waste (iron chloride, etc.) | 0.5 – 0.8 kg/kg |
Magnesium chloride (MgCl₂)
Input power (kWh/kg)
2 – 3 kWh/kg
(for concentration and crystallization from seawater or salt lake water)
- input fuel
Type: Natural gas or coke
Approximate quantity: 0.1 – 0.15 kg/kg
(Used in drying and concentration processes)
CO₂ emissions other than combustion (kg CO₂/kg)
0.01 – 0.03 kg CO₂/kg
(Byproducts from chemical reactions during concentration and processing)
- amount of change in resources such as soil, stone, and water (kg)
Seawater or salt lake water usage: 20 – 25 kg/kg
(Sources of magnesium salts)
- input item name and input amount guideline
Salt lake water containing magnesium: 20 – 25 kg/kg
Hydrochloric acid (HCl): 0.3 – 0.5 kg/kg
(Acid treatment accelerates dissolution reaction)
- process substances such as solvents
Name: Water
Usage: 10 – 15 kg/kg
Circulation rate: 80 – 90
(Reused in the concentration process)
- waste requiring special treatment
Name: Impurity-containing sediment
Approximate quantity: 0.1 – 0.2 kg/kg
(Calcium and other impurities)
- catalyst use and degradation rate
Catalyst: None
(No catalyst is required for this production process.)
Process Overview and Background
Manufacturing process: Magnesium obtained from seawater or salt lake water is treated with hydrochloric acid, concentrated and crystallized to produce magnesium chloride.
Energy consumption: electricity and fuel are required for drying and concentration.
Environmental Impact Management: Proper management of seawater use and waste is important, and impurity sediment treatment is necessary.
summary
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 2 – 3 kWh/kg |
| Fuel consumption | 0.1 – 0.15 kg/kg |
| CO₂ emissions other than combustion | 0.01 – 0.03 kg CO₂/kg |
| Salt lake water usage | 20 – 25 kg/kg |
| Hydrochloric acid usage | 0.3 – 0.5 kg/kg |
| Waste (sediment) | 0.1 – 0.2 kg/kg |
Triethylaluminum (TEAl)
Input power (kWh/kg)
5 – 8 kWh/kg
(Used for temperature control and stirring of reactions and in the purification process)
- input fuel
Type: Natural gas
Approximate quantity: 0.2 – 0.3 kg/kg
(used for heating and distillation)
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.05 – 0.1 kg CO₂/kg
(Byproducts of chemical reactions of raw materials)
- amount of change in resources such as soil, stone, and water (kg)
Alumina from bauxite: 5 – 7 kg/kg
(Supplied as aluminum concentrate)
- input item name and input amount guideline
Ethylene: 2.5 – 3.0 kg/kg
(Used for alkylation with aluminum)
Alumina (Al₂O₃): 1.5 – 2.0 kg/kg
(as a source of aluminum)
Hydrogen gas: 0.05 – 0.1 kg/kg
(Reaction acceleration and safety control)
- process substances such as solvents
Name: Hexane
Usage: 2.0 – 3.0 kg/kg
Circulation rate: 80 – 90
(Solvent for aluminum alkylation reaction)
- waste requiring special treatment
Name: Oxide waste (by-product alumina residue)
Approximate quantity: 0.1 – 0.2 kg/kg
(Impurity residue in the refining process)
- catalyst use and degradation rate
Catalyst: Triethylaluminum itself acts partly as a catalyst
Degradation rate: 0.01 – 0.02 kg per kg of production
Process Overview and Background
Production Process: Ethylene reacts with an aluminum source to proceed with the alkylation of aluminum. Hexane is commonly used as the solvent, and distillation is used for purification and separation.
Energy consumption: Temperature control and refining processes are the high energy consumption parts.
Waste management: Aluminum residues need to be reused and properly treated.
summary
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 5 – 8 kWh/kg |
| Fuel consumption | 0.2 – 0.3 kg/kg |
| CO₂ emissions other than combustion | 0.05 – 0.1 kg CO₂/kg |
| Alumina input | 1.5 – 2.0 kg/kg |
| Ethylene usage | 2.5 – 3.0 kg/kg |
| Solvent (hexane) usage | 2.0 – 3.0 kg/kg |
| waste | 0.1 – 0.2 kg/kg (oxides) |
Copper oxide (CuO)
Input power (kWh/kg)
1.5 – 3.0 kWh/kg
(Agitation, furnace temperature control, refining process)
- input fuel
Type: Natural gas
Quantity: 0.2 – 0.3 kg/kg
(For heating in firing furnaces)
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.02 – 0.05 kg CO₂/kg
(a byproduct of oxidation reactions and drying processes)
- amount of change in resources such as soil, stone, and water (kg)
Input from concentrate: 2.5 – 3.0 kg/kg
(copper concentrate with a copper content of about 30-40%)
- input item name and input amount guideline
Copper concentrate: 2.5 – 3.0 kg/kg
(Principal Component)
Oxygen (in air): 1.0 – 1.2 kg/kg
(Used in oxidation process)
- process substances such as solvents
Name: None (no solvent is used)
Circulation rate: N/A
- waste requiring special treatment
Name: Impurities (slag)
Approximate quantity: 0.1 – 0.2 kg/kg
- catalyst use and degradation rate
Catalyst: N/A (no catalyst used in oxidation process)
Process Overview
Manufacturing Process:
Copper concentrates are crushed and heated in a furnace to react with oxygen to produce CuO (copper oxide).
Heating temperatures are usually maintained around 900°C.
Impurities are generated as byproducts and are treated appropriately.
Energy consumption points:
Electricity and natural gas are consumed to heat and stir the furnace.
For efficiency, power control is emphasized while minimizing natural gas usage.
summary
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 1.5 – 3.0 kWh/kg |
| Fuel consumption (natural gas) | 0.2 – 0.3 kg/kg |
| CO₂ emissions other than combustion | 0.02 – 0.05 kg CO₂/kg |
| Input of concentrate | 2.5 – 3.0 kg/kg |
| Oxygen usage (in air) | 1.0 – 1.2 kg/kg |
| waste | 0.1 – 0.2 kg/kg (slag) |
Nickel catalyst (Ni catalyst)
Input power (kWh/kg)
2.5 – 4.0 kWh/kg
(electrolytic treatment, agitation, drying process)
- input fuel
Type: Natural gas
Quantity: 0.15 – 0.25 kg/kg
(Used in drying and reduction processes)
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.05 – 0.1 kg CO₂/kg
(chemical reactions and by-products of the formation process)
- amount of change in resources such as soil, stone, and water (kg)
Input from concentrate: 3.0 – 4.0 kg/kg
(average nickel concentrate content 15-25%)
- input item name and input amount guideline
Nickel concentrate: 3.0 – 4.0 kg/kg
Hydrogen: 0.05 – 0.1 kg/kg
(Used in catalyst reduction process)
Support material (e.g. alumina): 0.1 – 0.2 kg/kg
- process substances such as solvents
Name: Ammonia water
Usage: 0.5 – 1.0 kg/kg
Circulation rate: 60 – 70%.
- waste requiring special treatment
Name: Sludge (oxidation impurities)
Approximate quantity: 0.2 – 0.4 kg/kg
- catalyst degradation rate and usage guidelines
Catalyst usage: The product in question is a catalyst (Ni catalyst itself)
Degradation rate: 0.5 – 1.0%/kg (per use cycle)
Process Overview
Manufacturing Process:
Nickel concentrates are refined at high temperatures to extract nickel from oxides and sulfides.
Nickel metal is processed into a catalyst using hydrogen in the reduction process.
Surface area is improved by adhering to support materials such as alumina and silica.
Drying and finishing treatments optimize catalysts.
Energy consumption points:
The reduction and drying processes require a lot of energy, and natural gas and electricity are the main sources used.
summary
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 2.5 – 4.0 kWh/kg |
| Fuel consumption (natural gas) | 0.15 – 0.25 kg/kg |
| CO₂ emissions other than combustion | 0.05 – 0.1 kg CO₂/kg |
| Input of concentrate | 3.0 – 4.0 kg/kg |
| Hydrogen usage | 0.05 – 0.1 kg/kg |
| Support material (alumina, etc.) | 0.1 – 0.2 kg/kg |
| Solvent (ammonia water) | 0.5 – 1.0 kg/kg |
| Waste (sludge) | 0.2 – 0.4 kg/kg |
Ruthenium (Ru)
Input power (kWh/kg)
30 – 45 kWh/kg
(electrolysis, refining, melting, and other processes)
- input fuel
Type: Natural gas
Quantity: 0.5 – 0.8 kg/kg
(Used in refining and drying processes)
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.05 – 0.1 kg CO₂/kg
(Byproduct of chemical processing of concentrates)
- amount of change in resources such as soil, stone, and water (kg)
Input of concentrate: 200 – 300 kg/kg
(Ruthenium content is about 0.1-0.5%)
- input item name and input amount guideline
Ruthenium concentrate: 200 – 300 kg/kg
Hydrogen: 0.05 – 0.1 kg/kg
(Used in the reduction process)
Acids (e.g. nitric acid): 0.8 – 1.2 kg/kg
(Used in refining process)
- process substances such as solvents
Name: Ammonium hydroxide
Usage: 0.2 – 0.5 kg/kg
Circulation rate: 60 – 80
- waste requiring special treatment
Name: Chemical sludge (contained impurities)
Approximate quantity: 0.5 – 1.0 kg/kg
- catalyst degradation rate and usage guidelines
Catalyst usage: Used for some reactions in ore processing
Degradation rate: 1 – 2%/kg
Process Overview
Manufacturing Process:
Concentrates are extracted from ruthenium-bearing ores.
Dissolve with chemicals (e.g., nitric acid) and separate ruthenium.
Electrolysis or reduction process produces high-purity metallic ruthenium.
Fused or powdered as needed for use in catalysts and alloys.
Key energy consumption points:
High volume processing of concentrates and chemical refining processes use a lot of electricity and fuel.
summary
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 30 – 45 kWh/kg |
| Fuel consumption (natural gas) | 0.5 – 0.8 kg/kg |
| CO₂ emissions other than combustion | 0.05 – 0.1 kg CO₂/kg |
| Input of concentrate | 200 – 300 kg/kg |
| Hydrogen usage | 0.05 – 0.1 kg/kg |
| Acid (e.g., nitric acid) | 0.8 – 1.2 kg/kg |
| Solvent (ammonium hydroxide) | 0.2 – 0.5 kg/kg |
| Waste (chemical sludge) | 0.5 – 1.0 kg/kg |
Iodine (I₂)
Input power (kWh/kg)
3 – 5 kWh/kg
(Electricity consumption in the extraction and purification process)
- input fuel
Type: Natural gas
Quantity: 0.2 – 0.4 kg/kg
(Used in heating and concentration processes)
CO₂ emissions other than combustion (kg CO₂/kg)
0.01 – 0.05 kg CO₂/kg
(Occurs as a byproduct of chemical reactions)
- amount of change in resources such as soil, stone, and water (kg)
Iodine-containing concentrate input: 50 – 100 kg/kg
(for iodine concentrations of 0.5-2%)
- input item name and input amount guideline
Iodine-bearing concentrate: 50 – 100 kg/kg
Acid (sulfuric acid): 0.5 – 1.0 kg/kg
(Used for separation from iodide)
Oxidant (chlorine): 0.2 – 0.3 kg/kg
(Used for redox reactions)
- process substances such as solvents
Name: Water
Usage: 5 – 10 L/kg
Circulation rate: 70 – 85
(used for dissolution and purification)
- waste requiring special treatment
Name: Chemical sludge (insoluble residue)
Approximate quantity: 0.2 – 0.5 kg/kg
- catalyst degradation rate and usage guidelines
Catalyst usage: No or very little catalyst is used.
Degradation rate: N/A.
Process Overview
Extraction of concentrates:
Iodide is extracted from iodine-bearing ores (or groundwater).
Acid and oxidant treatment:
oxidizes iodide to iodine using sulfuric acid
and chlorine to form a precipitate.
Purification and concentration:
Dissolve in water and further purify and concentrate. Finally, iodine is recovered and crystallized.
summary
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 3 – 5 kWh/kg |
| Fuel consumption (natural gas) | 0.2 – 0.4 kg/kg |
| CO₂ emissions other than combustion | 0.01 – 0.05 kg CO₂/kg |
| Input of concentrate | 50 – 100 kg/kg |
| Acid (sulfuric acid) | 0.5 – 1.0 kg/kg |
| Oxidant (chlorine) | 0.2 – 0.3 kg/kg |
| Water usage | 5 – 10 L/kg |
| Waste (chemical sludge) | 0.2 – 0.5 kg/kg |
Ruthenium chloride (RuCl₃)
Input power (kWh/kg)
2 – 5 kWh/kg
(Used for dissolution, crystallization, and drying processes)
- input fuel
Type: Natural gas
Quantity: 0.2 – 0.3 kg/kg
(Used in drying process, etc.)
CO₂ emissions other than combustion (kg CO₂/kg)
0.01 – 0.02 kg CO₂/kg
(Byproducts of chemical reactions)
- amount of change in resources such as soil, stone, and water (kg)
na
(Manufactured from metallic ruthenium, not ore)
- input item name and input amount guideline
Ruthenium metal: 1.35 kg/kg
(Considering some losses in the process of converting ruthenium to 100%)
Hydrochloric acid (HCl): 1.2 – 1.5 kg/kg
(to convert ruthenium to chloride)
Chlorine gas (Cl₂): 0.05 – 0.1 kg/kg
(Used to promote oxidation and chlorination)
- process substances such as solvents
Name: Water
Usage: 3 – 6 L/kg
Circulation rate: 75 – 85
(used in reaction and cleaning processes)
- waste requiring special treatment
Name: Acidic liquid waste, impurity precipitation
Approximate quantity: 0.5 – 1.0 kg/kg
- catalyst degradation rate and usage guidelines
Catalyst: No catalyst or very small amount
Deterioration rate: N/A
Process Overview
Dissolution and Oxidation:
Metal ruthenium is oxidized using hydrochloric acid and chlorine gas to convert it to ruthenium chloride.
Concentration and crystallization:
The reaction solution is concentrated to precipitate and crystallize ruthenium chloride.
Washing and drying:
Crystals are washed and dried to obtain the final high-purity RuCl₃.
summary
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 2 – 5 kWh/kg |
| Fuel consumption (natural gas) | 0.2 – 0.3 kg/kg |
| CO₂ emissions other than combustion | 0.01 – 0.02 kg CO₂/kg |
| Ruthenium metal input | 1.35 kg/kg |
| Hydrochloric acid usage | 1.2 – 1.5 kg/kg |
| Chlorine usage | 0.05 – 0.1 kg/kg |
| Water usage | 3 – 6 L/kg |
| Waste (acidic liquid waste, etc.) | 0.5 – 1.0 kg/kg |
Acetonitrile (CH₃CN)
Input power (kWh/kg)
2 – 4 kWh/kg
(Energy required for distillation and refining processes)
- input fuel
Type: Natural gas or petroleum-based fuel
Quantity: 0.1 – 0.3 kg/kg
(Used in heating and drying process)
CO₂ emissions other than combustion (kg CO₂/kg)
0.05 – 0.1 kg CO₂/kg
(by processing chemical reactions and byproducts)
- amount of change in resources such as soil, stone, and water (kg)
na
(For production by organic synthesis)
- input item name and input amount guideline
Acetamide (CH₃CONH₂): 1.2 – 1.4 kg/kg
(precursor of acetonitrile)
Ammonium sulfate: 0.05 – 0.1 kg/kg
(stabilization of byproducts)
Sodium hydroxide (NaOH): 0.02 – 0.05 kg/kg
(for pH adjustment)
- process substances such as solvents
Name: Water
Usage: 2 – 4 L/kg
Circulation rate: 70 – 85
(used in the reaction and cooling process)
- waste requiring special treatment
Name: Sulfate Waste Liquid
Approximate quantity: 0.3 – 0.5 kg/kg
- catalyst degradation rate and usage guidelines
Catalyst: Acid catalyst (sulfuric acid)
Usage: 0.01 – 0.02 kg/kg
Degradation rate: 5 – 10%.
Process Overview
Dehydration of acetamide:
Dehydration of acetamide at high temperature to form acetonitrile. Acid catalysts are commonly used.
Purification and distillation:
The acetonitrile produced is distilled to increase its purity.
Wastewater treatment:
Appropriate treatment of sulfate generated as a byproduct.
summary
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 2 – 4 kWh/kg |
| Fuel consumption (natural gas, etc.) | 0.1 – 0.3 kg/kg |
| CO₂ emissions other than combustion | 0.05 – 0.1 kg CO₂/kg |
| Acetamide input | 1.2 – 1.4 kg/kg |
| Ammonium Sulfate Usage | 0.05 – 0.1 kg/kg |
| Water usage | 2 – 4 L/kg |
| Amount of acid catalyst used | 0.01 – 0.02 kg/kg |
| Waste (Sulfate waste) | 0.3 – 0.5 kg/kg |
Acrylamide (CH₂=CHCONH₂)
Input power (kWh/kg)
0.5 – 1.2 kWh/kg
(Used for pumps and cooling systems in reaction control and refining processes)
- input fuel
Type: Natural gas or heavy oil
Quantity: 0.05 – 0.1 kg/kg
(heating process to maintain reaction temperature)
CO₂ emissions other than combustion (kg CO₂/kg)
0.02 – 0.05 kg CO₂/kg
(derived from chemical reactions and byproducts)
- amount of change in resources such as soil, stone, and water (kg)
na
(Manufactured mainly by chemical synthesis)
- input item name and input amount guideline
Acrylonitrile (CH₂=CHCN): 1.1 – 1.2 kg/kg
(Main raw material)
Water: 3 – 5 L/kg
(for reaction media and cooling)
Copper sulfate catalyst: 0.01 – 0.02 kg/kg
(Copper-based catalysts are used in oxidation reactions)
- process substances such as solvents
Name: Water
Usage: 3 – 5 L/kg
Circulation rate: 80 – 90
(Reused for reaction and cooling processes)
- waste requiring special treatment
Name: Wastewater (including acrylonitrile residue)
Approximate quantity: 0.3 – 0.6 kg/kg
- catalyst degradation rate and usage guidelines
Catalyst: copper sulfate
Usage: 0.01 – 0.02 kg/kg
Degradation rate: 10 – 15
Process Overview
Hydration reaction of acrylonitrile:
Acrylonitrile is reacted with water to synthesize acrylamide using a copper oxide catalyst.
Cooling and Purification:
The product is cooled and the solvent is recirculated. Acrylamide is separated by distillation to achieve high purity.
Wastewater treatment:
Wastewater containing byproducts and unreacted materials is treated according to environmental standards.
summary
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.5 – 1.2 kWh/kg |
| Fuel consumption (natural gas, etc.) | 0.05 – 0.1 kg/kg |
| CO₂ emissions other than combustion | 0.02 – 0.05 kg CO₂/kg |
| Acrylonitrile input | 1.1 – 1.2 kg/kg |
| Water usage | 3 – 5 L/kg |
| Catalyst (copper sulfate) | 0.01 – 0.02 kg/kg |
| Catalyst degradation rate | 10 – 15% of |
| Waste (wastewater) | 0.3 – 0.6 kg/kg |
Potassium ore concentrate
Input power (kWh/kg)
0.8 – 1.5 kWh/kg
(used for crushing, flotation beneficiation and drying)
- input fuel
Type: Natural gas or heavy oil
Quantity: 0.05 – 0.1 kg/kg
(Used as a heat source in the drying process)
CO₂ emissions other than combustion (kg CO₂/kg)
0.02 – 0.05 kg CO₂/kg
(a byproduct of the reaction process)
- amount of change in resources such as soil, stone, and water (kg)
Waste rock and impurities: 2 – 4 kg/kg
(Separate unwanted components from crude ore)
- input item name and input amount guideline
Potash ore crude: 3 – 5 kg/kg
(potassium content 20 – 30%)
Water: 2 – 3 L/kg
(for flotation beneficiation and crushing)
- process substances such as solvents
Name: Flotation agent (surfactant, etc.)
Usage: 0.01 – 0.02 kg/kg
Circulation rate: 80 – 90
(Some of them can be reused.)
- waste requiring special treatment
Name: Waste mud, slurry
Approximate quantity: 1 – 2 kg/kg
- catalyst degradation rate and usage guidelines
Catalyst: not used (physical beneficiation is the main method)
Process Overview
Crushing and flotation beneficiation:
Crush the coarse ore and separate the potassium components as concentrate by flotation.
Drying and removal of impurities:
The wet concentrate is dried to make the final potash ore concentrate.
Waste Disposal:
Waste mud and impurities must be properly disposed of.
summary
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.8 – 1.5 kWh/kg |
| Fuel consumption (natural gas, etc.) | 0.05 – 0.1 kg/kg |
| CO₂ emissions other than combustion | 0.02 – 0.05 kg CO₂/kg |
| Crude ore input | 3 – 5 kg/kg |
| Water usage | 2 – 3 L/kg |
| Flotation agent usage | 0.01 – 0.02 kg/kg |
| Waste (waste mud, slurry) | 1 – 2 kg/kg |
palladium nitrate
Input power (kWh/kg)
0.8 – 1.5 kWh/kg
(for dissolution of palladium metal and reaction control)
- input fuel
Type: Natural gas or electric furnace power
Quantity: 0.05 – 0.1 kg/kg
(Heating applications in melting furnaces)
CO₂ emissions other than combustion (kg CO₂/kg)
0.01 – 0.05 kg CO₂/kg
(Byproduct of nitric acid reaction)
- amount of change in resources such as soil, stone, and water (kg)
Waste liquid: 1.2 – 2.0 kg/kg
(nitric acid residue and washing effluent after the reaction)
- input item name and input amount guideline
Palladium metal: 0.65 – 0.68 kg/kg
(Metal purity 99.95%)
Nitric acid: 0.4 – 0.5 kg/kg
(Required for oxidation reaction)
- process substances such as solvents
Name: Deionized water
Usage: 1.5 – 2.5 L/kg
Circulation rate: 85 – 95
(used for reaction and cleaning)
- waste requiring special treatment
Name: Nitric acid waste liquid
Approximate quantity: 1.0 – 1.8 kg/kg
(Neutralization treatment required)
- catalyst use and degradation rate
Use of catalysts: None (no catalysts are used in the production of palladium nitrate)
Process Overview
Dissolving Palladium Metal:
Pure palladium metal is dissolved in nitric acid to produce palladium nitrate.
Reaction and separation:
Impurities, if any, are removed by filtration process. Concentration and crystallization process.
Waste liquid treatment:
Waste liquid containing nitric acid should be neutralized and disposed of appropriately.
summary
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.8 – 1.5 kWh/kg |
| Fuel consumption (natural gas, etc.) | 0.05 – 0.1 kg/kg |
| CO₂ emissions other than combustion | 0.01 – 0.05 kg CO₂/kg |
| Palladium metal input | 0.65 – 0.68 kg/kg |
| Nitric acid usage | 0.4 – 0.5 kg/kg |
| Water usage | 1.5 – 2.5 L/kg |
| Nitric acid waste (waste) | 1.0 – 1.8 kg/kg |
important point
Care must be taken in handling nitric acid during the manufacturing process.
Waste treatment requires proper neutralization of acidic effluents and compliance with discharge standards.
This process simplifies inputs and improves efficiency over using ore, but requires risk management for nitric acid treatment.
alpha olefin
Input power (kWh/kg)
0.5 – 1.2 kWh/kg
(energy consumption for compression, reaction, distillation, etc. throughout the process)
- input fuel
Type: Natural gas (for process heating)
Quantity: 0.05 – 0.15 kg/kg
CO₂ emissions other than combustion (kg CO₂/kg)
0.01 – 0.05 kg CO₂/kg
(possible byproduct of process reactions CO₂)
- amount of change in resources such as soil, stone, and water (kg)
Water consumption: 2.0 – 3.0 kg/kg
(used for cooling and cleaning, dependent on circulation rate)
- input item name and input amount guideline
Ethylene: 1.05 – 1.1 kg/kg
(the main raw material for the production of alpha olefins)
Hydrogen: 0.01 – 0.02 kg/kg
(for reaction control)
- process substances such as solvents
Name: Deionized water
Usage: 2.0 – 3.0 L/kg
Circulation rate: 90
- waste requiring special treatment
Name: Reaction by-products (light olefins, etc.)
Approximate quantity: 0.02 – 0.05 kg/kg
- catalyst use and degradation rate
Catalyst type: Ziegler catalyst (aluminum-based)
Usage: 0.01 – 0.02 kg/kg
Catalyst degradation rate: 2 – 5%/kg production per catalyst
Process Overview
Polymerization of Ethylene:
Ethylene is converted to alpha-olefins through a polymerization reaction. This reaction primarily uses Ziegler catalysts.
Separation and Purification:
The product is purified by distillation to recover the desired carbon number of alpha olefin.
Waste Disposal:
Byproducts such as light olefins are generated, which are collected and reused or properly disposed of.
summary
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.5 – 1.2 kWh/kg |
| Fuel consumption (natural gas) | 0.05 – 0.15 kg/kg |
| CO₂ emissions other than combustion | 0.01 – 0.05 kg CO₂/kg |
| Ethylene input | 1.05 – 1.1 kg/kg |
| Hydrogen input | 0.01 – 0.02 kg/kg |
| Water usage | 2.0 – 3.0 L/kg |
| Byproducts (waste) | 0.02 – 0.05 kg/kg |
| Catalyst usage (Ziegler catalyst) | 0.01 – 0.02 kg/kg |
| Catalyst degradation rate | 2 – 5% / kg Production |
important point
Catalyst management and disposal is critical and requires an appropriate regeneration process.
Ethylene is highly reactive and requires a safe process design.
Such processes are one of the key raw materials in the polymer industry, and the efficiency of chemical reactions and the management of byproducts have a significant impact on production costs.
ethyl benzene
Input power (kWh/kg)
0.4 – 0.8 kWh/kg
(energy for compression, distillation, and reaction throughout the process)
- input fuel
Type: Natural gas (process heating)
Quantity: 0.05 – 0.1 kg/kg
CO₂ emissions other than combustion (kg CO₂/kg)
0.01 – 0.03 kg CO₂/kg
(Emissions from raw materials and byproducts)
- amount of change in resources such as soil, stone, and water (kg)
Water usage: 2.0 – 4.0 kg/kg
(cooling and cleaning applications)
- input item name and input amount guideline
Benzene: 0.65 – 0.7 kg/kg
Ethylene: 0.3 – 0.35 kg/kg
- process substances such as solvents
Name: Deionized water
Usage: 2.0 – 4.0 L/kg
Circulation rate: 85 – 95
- waste requiring special treatment
Name: Tar, unreacted hydrocarbons
Approximate quantity: 0.02 – 0.05 kg/kg
- catalyst use and degradation rate
Catalyst type: Aluminum chloride catalyst
Usage: 0.005 – 0.01 kg/kg
Degradation rate: 3 – 7% / kg Production
Process Overview
Alkylation reaction:
Benzene reacts with ethylene to form ethylbenzene. Aluminum chloride catalysts are commonly used for this reaction.
Distillation and Purification:
The ethylbenzene produced is distilled to separate byproducts and unreacted material.
Waste management:
Tar and unreacted hydrocarbons as byproducts are properly recovered and disposed of.
summary
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.4 – 0.8 kWh/kg |
| Fuel consumption (natural gas) | 0.05 – 0.1 kg/kg |
| CO₂ emissions other than combustion | 0.01 – 0.03 kg CO₂/kg |
| Benzene input | 0.65 – 0.7 kg/kg |
| Ethylene input | 0.3 – 0.35 kg/kg |
| Water usage | 2.0 – 4.0 L/kg |
| Byproducts (waste) | 0.02 – 0.05 kg/kg |
| Catalyst usage | 0.005 – 0.01 kg/kg |
| Catalyst degradation rate | 3 – 7% / kg Production |
important point
The use of benzene requires compliance with safety standards and the process must be sealed.
Catalysts used are regularly collected and recycled to reduce costs and environmental impact.
This process is widely used as an important intermediate step in the production of compounds such as styrene monomers.
Seeds for seeding
Input power (kWh/kg)
0.1 – 0.3 kWh/kg
(for seed sorting, drying, processing, and packaging)
- input fuel
Type: Natural gas or diesel fuel (heater for drying)
Quantity: 0.02 – 0.05 kg/kg
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.005 – 0.02 kg CO₂/kg
(indirect emissions during transportation and processing)
- amount of change in resources such as soil, stone, and water (kg)
Water consumption: 5 – 10 kg/kg
(for cleaning and treating seed for sowing)
- input item name and input amount guideline
Raw seed: 1.5 – 2.0 kg/kg
Germination stimulant: 0.01 – 0.03 kg/kg
Examples: Acid treatment agents, zinc sulfate, etc.
- process substances such as solvents
Name: Water, germination treatment chemicals (e.g., gibberellin)
Usage: 0.5 – 1.0 L/kg
Circulation rate: 80 – 90
- waste requiring special treatment
Name: Ungerminated and defective seeds
Approximate quantity: 0.05 – 0.1 kg/kg
- catalyst use and degradation rate
Catalyst type: germination stimulants such as gibberellins
Usage: 0.01 – 0.02 kg/kg
Degradation rate: 10 – 15% / kg production
Process Overview
Raw material preparation:
After harvesting, the raw material seeds are cleaned, dried, and chemically treated to facilitate germination.
Germination enhancement treatment:
Zinc sulfate and gibberellin are used on seeds to accelerate germination and improve quality.
Sorting and packaging:
Defective seeds are sorted and only good seeds are processed and packaged.
summary
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.1 – 0.3 kWh/kg |
| Fuel consumption (natural gas and diesel) | 0.02 – 0.05 kg/kg |
| CO₂ emissions other than combustion | 0.005 – 0.02 kg CO₂/kg |
| Raw seed input | 1.5 – 2.0 kg/kg |
| Germination accelerator | 0.01 – 0.03 kg/kg |
| Water consumption | 5 – 10 L/kg |
| Waste (ungerminated and defective seeds) | 0.05 – 0.1 kg/kg |
| Catalyst usage (e.g., gibberellins) | 0.01 – 0.02 kg/kg |
| Catalyst degradation rate | 10 – 15% / kg Production |
important point
The germination accelerators used must be properly managed, with ecological impacts taken into consideration.
It is recommended that defective seeds generated as waste be reused as a raw material for fertilizer or biomass energy.
This process is critical to providing high quality seed for agricultural applications.
seed
Input power (kWh/kg)
0.05 – 0.15 kWh/kg
Electric power for seed sorting, drying, and cooling storage
- input fuel
Type: Diesel fuel (for agricultural machinery and transportation)
Quantity: 0.02 – 0.08 kg/kg
3 CO₂ emissions other than combustion (kg CO₂/kg)
0.01 – 0.02 kg CO₂/kg
Indirect emissions from transportation and chemical processing
- amount of change in resources such as soil, stone, and water (kg)
Water: 5 – 15 L/kg
Used for seed cleaning, germination testing and processing
- input item name and input amount guideline
Raw material plant: 1.2 – 2.0 kg/kg
Raw materials grown on farmland (e.g., rice, corn, cotton, rapeseed, etc.)
Germination promoter/fertilizer: 0.005 – 0.02 kg/kg
Examples: potassium nitrate, phosphate
- process substances such as solvents
Name: Water, dilute acid solution (for acid treatment)
Usage: 0.2 – 1.0 L/kg
Circulation rate: 70 – 90
- waste requiring special treatment
Name: Ungerminated seeds, defective
Approximate quantity: 0.05 – 0.1 kg/kg
- catalyst use and degradation rate
Catalyst type: phosphate or gibberellin (as germination promoter)
Usage: 0.01 – 0.03 kg/kg
Degradation rate: 10 – 20% / kg Production
Process Overview
Cultivation and harvesting:
After cultivation on the farm, seeds are extracted from the harvested plants.
Cleaning and sorting:
The seed is cleaned and the best seed is sorted. Immature or defective products are eliminated.
Drying/treatment:
Dried to the appropriate moisture content and treated to enhance shelf life.
Germination promotion and testing:
Use germination promoters as needed to check germination rates.
Packaging and transportation:
Packaging the final product and preparing it for shipment.
summary
| (data) item | Numerical value (approx/kg) |
| electricity consumption | 0.05 – 0.15 kWh/kg |
| Fuel consumption (diesel) | 0.02 – 0.08 kg/kg |
| CO₂ emissions other than combustion | 0.01 – 0.02 kg CO₂/kg |
| Raw material plant input | 1.2 – 2.0 kg/kg |
| Germination promoters and fertilizers | 0.005 – 0.02 kg/kg |
| Water consumption | 5 – 15 L/kg |
| Waste (ungerminated and defective seeds) | 0.05 – 0.1 kg/kg |
| Catalyst usage (gibberellins, etc.) | 0.01 – 0.03 kg/kg |
| Catalyst degradation rate | 10 – 20% / kg Production |
important point
It is recommended to increase the efficiency of water use on the farm and during processing to reduce environmental impact.
Waste seeds are reused as biofuel or compost to promote resource recycling.
This information applies to industrial seeding and commercial seed production processes.
agrochemical
The major active ingredients and their **Market Share Percentage (approximate)** worldwide. This includes herbicides, insecticides, and fungicides.
Pesticide Composition and Share Ratio (approximate)
| category | Examples of Ingredients | use | Share (%) |
| herbicide | Glyphosate | Weed suppression | 30 – 35% of |
| Atrazine | Broadleaf weed control | 10 – 15% of | |
| Paraquat | non-selective weeding | 5 – 10% | |
| Metolachlor | Suppression of weed germination | 5 – 8% | |
| pesticide | Imidacloprid | Control of sucking pests | 15 – 20%. |
| Chlorpyrifos | Extensive pest control | 10 – 15% of | |
| Pyrethroids | contact toxic insecticide | 8 – 12%, and | |
| Fipronil | pest control | 5 – 10% | |
| disinfectant | Mancozeb | Fungal Prevention | 10 – 12% (1.0 – 1.2) |
| Chlorothalonil | Extensive fungal eradication | 8 – 10% | |
| Azole-based | Treatment of fungal diseases | 10 – 15 | |
| Strobilurins | Resistant Fungal Control | 5 – 10% |
Description and Market Trends
herbicide
Glyphosate holds the largest share of the herbicide market. It is used as a non-selective herbicide, primarily for weed management in genetically modified crops.
Paraquat is fast-acting and used for weed control in non-crop areas, but is increasingly regulated due to toxicity.
pesticide
Imidacloprid is a representative neonicotinoid insecticide that is highly effective against sucking pests, but there are concerns about its effects on honeybees.
Pyrethroids are relatively safe insecticides used in a wide range of household and agricultural applications.
disinfectant
Mancozeb and chlorothalonil are widely used as prophylactics for fungal control.
Azoles and strobilurins are effective for both treatment and prevention, and are also useful in fruit cultivation.
Conclusion.
In the agrochemical market, herbicides account for about 50% of the total, followed by insecticides and fungicides, which have a large share of the market. While certain ingredients (such as glyphosate and imidacloprid) dominate some markets, new chemicals and organic pesticides are being developed in response to regulations and environmental impacts.
The shares of herbicides, insecticides, and fungicides in the agrochemical market are roughly as follows
Herbicide: 50 – 55
Insecticides: 25 – 30%
Fungicide: 15 – 20
breakdown explanation
Herbicide (50 – 55%)
Herbicides account for the largest share of the agrochemical market. This is mainly due to the high need for weed management in grain production.
Insecticide (25 – 30%)
Insecticides are used primarily to prevent damage to crops by pests and are especially important in the cultivation of fruits and vegetables.
Fungicide (15 – 20%)
Fungicides are used specifically to prevent fungal blight. They are more commonly used in humid areas and orchards.
This share distribution varies by region and crop type, but the overall market trend is this percentage.
MTBE (Methyl Tertiary Butyl Ether)
- input power
0.3 – 0.5 kWh/kg
- input fuel
Natural gas: 0.02 – 0.05 kg
Propane: 0.01 – 0.03 kg
CO₂ emissions other than combustion
0.05 – 0.08 kg CO₂/kg MTBE
- impacts on resources such as earth, rocks, and water
Water usage: 2 – 5 kg
Industrial cooling water: 80 – 90% circulation
- input
Isobutylene: 0.75 kg
Methanol: 0.35 kg
- process substances such as solvents
Hexane (process solvent): 0.02 kg, 90% circulation
Sulfuric acid (catalyst): 0.01 kg, degradation rate 5%/kg MTBE
- waste requiring special treatment
Waste catalyst (sulfate): 0.005 kg
Organic waste containing: 0.01 – 0.02 kg
Manufacturing Process Overview
MTBE is produced by the reaction of isobutylene and methanol. Sulfuric acid is used as a catalyst and the reaction proceeds under appropriate temperature and pressure. Cooling water is used in the circulation system and the solvent hexane is used in some processes.
In this process, the distillation and separation steps are primarily energy intensive, with MTBE ultimately being purified. Energy efficiency is managed through a balance between combustion energy and electricity.
Phosphate ore concentrate
- input power
0.4 – 0.7 kWh/kg concentrate
- input fuel
Fuel oil: 0.01 – 0.02 kg
Diesel: 0.02 – 0.05 kg (for mining equipment)
CO₂ emissions other than combustion
0.03 – 0.05 kg CO₂/kg concentrate
- impacts on resources such as earth, rocks, and water
Water usage: 1 – 3 kg
Waste rock and tailings: 4 – 6 kg (sediment and impurities)
- input item name and input amount guideline
Mined crude ore: 3 – 5 kg (approx. 30% phosphoric acid content)
Acid (beneficiation): sulfuric acid 0.01 – 0.02 kg
- process substances such as solvents
Flotation chemicals (e.g. anionic chemicals): 0.002 – 0.005 kg, 90% circulation
- waste requiring special treatment
Tailings (unreacted ore/impurities): 4 – 6 kg
Process wastewater: 0.5 – 1.5 kg
Manufacturing Process Overview
Phosphate concentrates are obtained from crude ores containing phosphate. The mined ore is crushed and the concentrate is obtained through a beneficiation process (flotation or gravity separation). The phosphate content of this concentrate is about **30-40%**. There are many impurities and untreated minerals that remain as tailings, and wastewater treatment is also required.
Energy consumption is used primarily in machinery for crushing and flotation, and acid treatment efficiently extracts phosphorus.
Calcium oxide (CaO)
- input power
0.5 – 1.0 kWh/kg CaO
- input fuel
Coal: 0.15 – 0.25 kg
Natural gas: 0.06 – 0.1 kg
CO₂ emissions other than combustion
0.75 – 0.85 kg CO₂/kg CaO (calcination reaction from limestone)
- impacts on resources such as earth, rocks, and water
Water consumption: 0.5 – 1.0 kg (for cooling)
Waste/tailings: 0.1 – 0.2 kg (ore impurities)
- input item name and input amount guideline
Limestone (CaCO₃): 1.8 – 2.0 kg (approx. 50-55% CaO content)
- process substances such as solvents
None (typically no solvent is used)
- waste requiring special treatment
Unreacted limestone/tailings: 0.1 – 0.2 kg
Process wastewater: 0.2 – 0.5 kg (after cooling)
Manufacturing Process Overview
Calcium oxide (CaO, calcined lime) is obtained by calcining limestone (CaCO₃) at high temperatures (about 900-1000°C). The process involves a calcination reaction that removes CO₂ from CaCO₃, resulting in the emission of about 0.8 kg of CO₂. Coal and natural gas are often used as energy sources, while electricity is consumed for crushing and transportation.
As waste, unreacted ores and trace impurities may be generated, and small amounts of wastewater may be produced in the cooling process.
Molybdenum concentrate
- input power
0.3 – 0.6 kWh/kg concentrate
- input fuel
Diesel fuel (used for mining and hauling): 0.4 – 0.6 kg
Natural gas (used in drying process): 0.05 – 0.1 kg
CO₂ emissions other than combustion
0.01 – 0.03 kg CO₂/kg (indirect emissions such as chemical reactions)
- impacts on resources such as earth, rocks, and water
Mined crude ore: 100 – 200 kg (molybdenum content in crude ore 0.5 – 1.0%)
Water consumption: 10 – 15 kg (used in the flotation beneficiation process)
- input item name and input amount guideline
Crude ore (molybdenum ore, e.g. molybdenite – MoS₂): 100 – 200 kg
Flotation agent (e.g., hydroxylate, silica floss, etc.): 0.1 – 0.2 kg
- process substances such as solvents
Defoamer: 0.05 – 0.1 kg (circulation rate: 80 – 90%)
Flocculant (e.g. polymer flocculant): 0.05 – 0.1 kg (circulation rate: 90%)
- waste requiring special treatment
Tailings (impurity-bearing minerals): 99 – 199 kg
Process wastewater (requires reprocessing): 5 – 10 kg
Manufacturing Process Overview
The production of molybdenum concentrates involves a process of beneficiation of molybdenum minerals (mainly molybdenite, MoS₂) from the crude ore. Flotation is the most common method, in which the ore is crushed and chemicals are used to separate the molybdenum from the other minerals. Flotation beneficiation uses large amounts of water and generates large amounts of impurities as tailings.
Light oil is used for mining and ore transport, while electricity is consumed primarily in the crushing and drying processes. Catalysts are not required, but the use of beneficiation chemicals generates process wastewater that must be properly treated.
Bismuth concentrate
- input power
0.4 – 0.8 kWh/kg Ore concentrate (operation of crushing and beneficiation facilities)
- input fuel
Diesel fuel (for mining and hauling): 0.5 – 0.7 kg
Natural gas (used in drying process): 0.05 – 0.1 kg
CO₂ emissions other than combustion
0.02 – 0.05 kg CO₂/kg (e.g. reaction of flotation beneficiation chemicals)
- impacts on resources such as earth, rocks, and water
Mined Crude Ore Volume: 50 – 100 kg (Bismuth content in Crude Ore: 1 – 2%)
Water consumption: 8 – 12 kg (used for flotation beneficiation)
- input item name and input amount guideline
Crude ore (typical example: sulfide ore containing bismuth, Bi₂S₃): 50 – 100 kg
Flotation agent (e.g. copper sulfate, xylene, etc.): 0.2 – 0.5 kg
- process substances such as solvents
Defoamer: 0.05 kg (circulation: 85%)
Flocculant (e.g. polymer flocculant): 0.05 – 0.1 kg (circulation rate: 90%)
- waste requiring special treatment
Tailings (residual minerals): 45 – 95 kg
Process wastewater (needs treatment): 5 – 8 kg
Manufacturing Process Overview
Bismuth concentrate is produced by separating crude ore containing mainly sulfide minerals (bismuth ore) using a flotation process. Due to the low concentration of bismuth, a large amount of coarse ore is required. The beneficiation process involves crushing and flotation beneficiation and requires the input of water and chemicals.
Light oil is used for mining and hauling, and electricity is used for crushing and beneficiation machine operation. Impurities remaining as tailings are discarded and the process effluent is reprocessed. Due to the low content of bismuth ore, it is essential to have an efficient mineralization process by beneficiation.
zinc concentrate
- input power
0.6 – 1.2 kWh/kg Ore concentrate (crushing and flotation beneficiation unit operation)
- input fuel
Diesel fuel (for mining and hauling): 0.4 – 0.6 kg
Natural gas (drying process): 0.05 – 0.1 kg
CO₂ emissions other than combustion
0.03 – 0.08 kg CO₂/kg (beneficiation chemicals and chemical treatment reactions)
- impacts on resources such as earth, rocks, and water
Mining Crude Ore Volume: 20 – 30 kg (Zinc content in Crude Ore: 3 – 5%)
Water consumption: 10 – 15 kg (flotation beneficiation process)
- input item name and input amount guideline
Crude ore (e.g. zinc ore, zinc flash ore ZnS): 20 – 30 kg
Flotation agent (e.g., xylene, copper sulfate): 0.1 – 0.2 kg
- process substances such as solvents
Flocculant: 0.05 kg (circulation rate: 85%)
Defoamer: 0.02 – 0.05 kg (circulation: 90%)
- waste requiring special treatment
Tailings: 18 – 28 kg
Process wastewater: 8 – 12 kg
Manufacturing Process Overview
Zinc-containing ores (mainly sphalerite ZnS) are used in the production of zinc concentrates. The mined ore is crushed and the zinc-containing component is separated as a concentrate by flotation. The zinc content in the crude ore is 3-5%, so a large amount of ore must be beneficiated from the crude ore.
Fuel is used for mining, transportation, and drying, while electricity is needed to operate the crushing and beneficiation equipment. The chemicals used make flotation beneficiation more efficient, and impurities that remain as tailings are properly disposed of. Wastewater treatment is also required, and some of the spent solvents and chemicals are reused.
Silica ore concentrate
- input power
0.5 – 1.0 kWh/kg Ore concentrate (crushing and beneficiation equipment operation)
- input fuel
Diesel fuel (mining and transportation): 0.3 – 0.6 kg
Natural gas (drying process): 0.05 – 0.08 kg
CO₂ emissions other than combustion
0.02 – 0.05 kg CO₂/kg (from chemical use and chemical processing)
- impacts on resources such as earth, rocks, and water
Mining Crude Ore Volume: 5 – 10 kg (Silica content in crude ore: 10 – 20%)
Water consumption: 3 – 5 kg (flotation beneficiation and washing process)
- input item name and input amount guideline
Crude ore (e.g. quartz ore SiO₂): 5 – 10 kg
Flotation agent (e.g., flow spar, sodium sulfate): 0.05 – 0.1 kg
- process substances such as solvents
Flocculant: 0.02 kg (circulation rate: 85%)
Defoamer: 0.01 – 0.03 kg (circulation: 90%)
- waste requiring special treatment
Tailings: 4 – 8 kg (impurities and unused mineral components)
Process wastewater: 2 – 4 kg
Manufacturing Process Overview
In the production of silica ore concentrate, ores containing mainly quartz (SiO₂) are mined. The mined ore is crushed to obtain high purity silica components by flotation beneficiation and washing. Since the silica content in the mined ore is 10 – 20%, concentrates must be obtained from a large amount of coarse ore.
Electricity and fuel are consumed in crushing, beneficiation, and drying. Chemical consumption is low, and some flocculants and defoamers are recycled. Tailings generated after beneficiation are disposed of, and wastewater must be properly treated.
Potassium salt ore concentrate
- input power
0.8 – 1.5 kWh/kg Ore concentrate (crushing and flotation equipment)
- input fuel
Diesel fuel (mining and transportation): 0.2 – 0.4 kg
Natural gas (drying process): 0.1 – 0.15 kg
CO₂ emissions other than combustion
0.05 – 0.1 kg CO₂/kg (chemical use and process emissions)
- impacts on resources such as earth, rocks, and water
Mined Crude Ore Volume: 6 – 8 kg (15 – 20% potassium content in crude ore)
Water consumption: 2 – 3 kg (used in beneficiation and washing processes)
- input item name and input amount guideline
Crude ore (e.g., carnallite, silvinite): 6 – 8 kg
Flotation agent (e.g., sulfuric acid, fatty acid derivatives): 0.02 – 0.05 kg
- process substances such as solvents
Flocculant: 0.02 kg (circulation rate: 80%)
Defoamer: 0.01 – 0.03 kg (circulation rate: 85%)
- waste requiring special treatment
Tailings (impurities): 5 – 7 kg (unwanted salt and gypsum components)
Process wastewater: 2 – 3 kg (needs treatment due to high salt concentration)
Manufacturing Process Overview
Potassium salt ore concentrates are produced from ores such as carnallite and silvinite. After the ores are crushed, impurities are removed by flotation beneficiation to obtain concentrates with a higher potassium concentration. Since the potassium content in the crude ore is around 15 – 20%, it is necessary to produce concentrates from a large amount of crude ore.
The beneficiation and washing processes require water, and flocculants and defoamers are also used as part of the process. Some of these agents are reused, but a large amount of waste is generated as tailings, and wastewater must also be treated properly.
Cobalt ore concentrate
- input power
4 – 6 kWh/kg Ore concentrate (crushing, flotation)
- input fuel
Diesel fuel (mining and transportation): 0.3 – 0.5 kg
Natural gas (drying process): 0.1 – 0.15 kg
CO₂ emissions other than combustion
0.1 – 0.15 kg CO₂/kg concentrate (chemical use, process origin)
- changes to resources such as earth, rocks, and water
Mined Crude Ore Volume: 20 – 30 kg (cobalt content 0.1 – 0.5%)
Water consumption: 5 – 8 kg (used for flotation and washing processes)
- input item name and input amount guideline
Crude ore (e.g. copper and nickel ores with cobalt): 20 – 30 kg
Flotation agent (e.g. sodium sulfide, xylates): 0.05 – 0.1 kg
- process substances such as solvents
Flocculant: 0.02 – 0.05 kg (circulation rate: 70 – 80%)
Defoamer: 0.01 kg (circulation: 85%)
- waste requiring special treatment
Tailings: 15 – 25 kg (ore residue with impurities)
Process wastewater: 5 – 8 kg (needs treatment due to heavy metal content)
Manufacturing Process Overview
The production of cobalt ore concentrates uses low-grade cobalt ore, which is contained with copper and nickel. The ore is crushed and impurities are removed by flotation to obtain a concentrate with a high cobalt concentration. Due to the low grade of the ore, it is necessary to obtain 1 kg of cobalt concentrate from approximately 20 – 30 kg of coarse ore.
In the beneficiation process, flocculants and defoamers are used, and some chemicals are reused. After beneficiation, a large amount of tailings is generated and wastewater must be treated.
Manganese oxide catalyst
- input power
3 – 5 kWh/kg
- input fuel
Natural gas: 0.2 – 0.3 kg (used in drying and firing processes)
Diesel fuel: 0.1 – 0.2 kg (transport and machine operation)
3.** Other than combustion CO
Other than combustion, CO₂
0.05 – 0.1 kg CO₂/kg (process origin, solvent decomposition)
- the amount of change to resources such as earth, rocks, and water
Feed of concentrate: 3 – 5 kg (manganese content 30 – 50%)
Water consumption: 4 – 6 kg (for cleaning and refining)
- input item name and input amount guideline
Manganese concentrate (MnO₂ content 30 – 50%): 3 – 5 kg
Nitric acid: 0.1 – 0.2 kg (oxidation promoted)
Potassium salts: 0.05 – 0.1 kg (activator)
- process substances such as solvents
Ion exchange water: 2 – 4 kg (circulation rate: 85%)
Organic solvent (e.g. methanol): 0.05 – 0.1 kg (circulation rate: 70%)
- waste requiring special treatment
Residue: 1 – 2 kg (including impurities and unreacted components)
Wastewater: 3 – 5 kg (contains heavy metals, requires treatment)
- catalyst degradation rate
Catalyst degradation rate: 0.01 – 0.02 kg/kg (during production process)
Manufacturing Process Overview
Manganese oxide catalysts are produced by oxidizing and calcining high-purity manganese concentrates. Approximately 3 – 5 kg of manganese concentrate is required, and solvents and water are used for purification. Nitric acid and potassium salts are used to promote oxidation and activation. The catalyst has a low degradation rate and can be mostly reused, although some wastewater and residues must be treated.
Copper catalyst
- input power
5 – 7 kWh/kg
- input fuel
Natural gas: 0.3 – 0.4 kg (for firing and drying)
Diesel fuel: 0.1 – 0.2 kg (transport or machine operation)
- other than combustion CO
0.1 – 0.15 kg CO₂/kg (from chemical processes and solvents)
- the amount of change to resources such as earth, rocks, and water
Copper concentrate input: 3 – 5 kg (20 – 30% copper content)
Water consumption: 5 – 7 kg (washing and solvent recovery)
- input item name and input amount guideline
Copper concentrate (20 – 30% copper content): 3 – 5 kg
Aluminum oxide: 0.2 – 0.3 kg (support)
Nitric acid: 0.1 – 0.2 kg (for oxidation process)
- process substances such as solvents
Ion exchange water: 3 – 5 kg (circulation rate: 85%)
Organic solvent (e.g. ethanol): 0.05 – 0.1 kg (circulation rate: 75%)
- waste requiring special treatment
Residue with impurities: 1 – 2 kg (requires appropriate treatment)
Wastewater containing heavy metals: 2 – 3 kg (requires wastewater treatment plant)
- catalyst degradation rate
Catalyst degradation rate: 0.02 – 0.03 kg/kg (renewal after long-term use)
Manufacturing Process Overview
Copper catalysts are formed mainly by oxidizing copper extracted from copper concentrates, together with aluminum oxide and other supports. Large amounts of water are used in the cleaning process, some of which is recycled, but some wastewater must be properly treated because it contains heavy metals. To increase energy efficiency, natural gas is used for drying and calcination. The catalyst has a low degradation rate and can be recycled, but some replacement is required.
Solvent for cobalt extraction
- input power
4 – 6 kWh/kg
- input fuel
Natural gas: 0.2 – 0.3 kg (for reaction and drying)
Diesel fuel: 0.1 kg (for transport or heating)
3 CO₂ emissions other than combustion
0.1 – 0.12 kg CO₂/kg (by-product from chemical reaction)
- the amount of change to resources such as earth, rocks, and water
Water consumption: 5 – 7 kg (solvent purification and washing)
- input item name and input amount guideline
Organic acid (e.g. decanoic acid): 0.3 – 0.4 kg
Alcohols (e.g. octanol): 0.3 – 0.5 kg
Surfactant: 0.05 – 0.1 kg
- process substances such as solvents
Ion exchange water: 3 – 4 kg (circulation rate: 85%)
Organic solvent (e.g. kerosene): 0.8 – 1 kg (circulation rate: 70%)
- waste requiring special treatment
Effluent (including organic effluent): 1.5 – 2 kg (requires proper treatment)
Residue with impurities: 0.2 – 0.3 kg
- catalyst degradation rate (if used)
Catalyst degradation rate: 0.01 – 0.02 kg/kg (if catalyst regeneration/replenishment is required)
Manufacturing Process Overview
Organic acids, alcohols, and sometimes surfactants are used to produce solvents for cobalt extraction to increase extraction efficiency. Hydrocarbon solvents such as kerosene are also added, many of which are recycled. During the production process, water is used for purification, and some wastewater must be properly treated because it contains organic matter. In addition, some CO₂ is by-produced during the chemical reaction process. Natural gas is used for reactions at high temperatures. If catalysts are used, their degradation rate is also taken into account and regeneration is performed.
barium sulfate concentrate
- input power
2.5 – 4.0 kWh/kg (used for crushing, beneficiation and concentration)
- input fuel
Diesel oil: 0.1 – 0.2 kg (for mechanical operation, transportation)
Natural gas: 0.2 – 0.3 kg (for drying and heating)
3 CO₂ emissions other than combustion
0.05 – 0.08 kg CO₂/kg (from chemical reactions and by-product gases)
- the amount of change to resources such as earth, rocks, and water
Water consumption: 3 – 5 kg (used for washing and flotation beneficiation)
Volume of earth excavated: 3 – 5 kg (barium concentration in ore: 30 – 50%)
- input item name and input amount guideline
Barite ore (BaSO₄ containing ore): 2 – 3 kg (as 30-50% content)
Limestone (CaCO₃): 0.05 – 0.1 kg (to accelerate separation)
- process substances such as solvents
Flotation agent (organic chemicals): 0.005 – 0.01 kg (circulation rate: 85%)
Water for cleaning: 4 – 5 kg (circulation rate: 90%)
- waste requiring special treatment
Waste mud (sludge with impurities): 0.3 – 0.5 kg
Mine waste rock: 2 – 3 kg
- catalyst degradation rate (if used)
Catalyst degradation rate: 0.001 – 0.005 kg/kg (regeneration and replenishment when necessary)
Manufacturing Process Overview
To produce barium sulfate (BaSO₄) concentrate, barite ore is mainly crushed, separated and concentrated using a flotation process. Depending on the barium content in the ore, 2 to 3 kg of barite is required. Impurities are removed by flotation beneficiation using a flotation agent, and waste mud is produced. The process water is circulated, but some of the discharged wastewater contains impurities and must be treated appropriately.
Catalysts are used in a few cases, but in some cases limestone is added to accelerate separation. Electricity and fuel are used for machine operation, drying, and transportation.
ilmenite concentrate
- input power
2.5 – 4.5 kWh/kg (used for crushing, magnetic separation, flotation)
- input fuel
Diesel oil: 0.15 – 0.25 kg (for mining, machine operation and transportation)
Natural gas: 0.3 – 0.4 kg (for drying and firing)
3 CO₂ emissions other than combustion
0.05 – 0.08 kg CO₂/kg (side reaction during impurity removal)
- the amount of change to resources such as earth, rocks, and water
Water consumption: 4 – 6 kg (used for flotation treatment and washing)
Volume of earth and rock drilled: 4 – 6 kg (ilmenite content: 30 – 50%)
- input item name and input amount guideline
Ilmenite-bearing ore: 2 – 3 kg (30 – 50% TiO₂ content)
Soda ash (Na₂CO₃): 0.05 – 0.1 kg (to accelerate separation)
- process substances such as solvents
Flotation agent (fatty acid-based chemicals): 0.005 – 0.01 kg (circulation rate: 85%)
Process water: 5 – 6 kg (circulation rate: 90%)
- waste requiring special treatment
Waste mud (sludge with impurities): 0.4 – 0.6 kg
Mine waste rock: 3 – 4 kg
- catalyst degradation rate (if used)
Catalyst degradation rate: 0.002 – 0.005 kg/kg (degradation depending on regeneration frequency)
Manufacturing Process Overview
In the production of ilmenite concentrate, concentrates are separated from titanium-bearing ores mainly by magnetic and flotation methods. Depending on the ilmenite content in the ore, 2 to 3 kg of raw ore is required. Fatty acid chemicals are used as flotation agents to remove impurities. Soda ash may be added for separation, and mine waste rock or waste mud is produced after processing. Although the process water is recycled, some of the treated water is disposed of and must be properly managed.
Energy is used primarily for grinding, drying, and transportation, while catalyst degradation is managed by replenishment as needed.
saltwater
- input power
0.05 – 0.1 kWh/kg (pumped transport and evaporation pond management)
- input fuel
Diesel fuel: 0.01 – 0.02 kg (used for pump and vehicle operation)
3 CO₂ emissions other than combustion
0.01 kg CO₂/kg (due to side reactions during dissolution and water sampling processes)
- the amount of change to resources such as earth, rocks, and water
Water loss by evaporation: 5 – 10 kg (natural evaporation to facilitate concentration)
Land use change: 0.1 – 0.2 m²/kg (evaporation pond maintenance)
- input item name and input amount guideline
Salt lake water (raw water): 3 – 5 kg (final volume obtained after concentration and precipitation)
Flocculant (polymer-based): 0.001 – 0.005 kg (for solids removal)
- process substances such as solvents
Process water: 0.2 – 0.5 kg (circulation rate for washing and pumping: 90%)
- waste requiring special treatment
Sediment (impurity sludge): 0.01 – 0.02 kg
Wastewater: 0.5 – 1 kg (non-recycled portion)
- catalyst degradation rate (if used)
Catalyst degradation rate: 0.001 – 0.002 kg/kg (coagulant degradation and restocking)
Process Overview
The most common process for acquiring salt lake water is to collect raw water from a salt lake and naturally concentrate it in an evaporation pond. Small amounts of electricity and fuel are used for pumping and transportation. Extensive evaporation pond maintenance is required during the concentration process, as large amounts of water are lost through natural evaporation. Polymer chemicals such as flocculants are used, and sediment and sludge are generated as waste.
In this process, it is important to increase water reuse rates and properly manage wastewater treatment. In addition, flocculants and catalysts degrade in small amounts, but must be continuously replenished.
copper concentrate
- input power
2 – 4 kWh/kg
(mining, crushing, flotation process)
- input fuel
Diesel fuel: 0.1 – 0.3 kg
(for mining equipment, trucking)
3 CO₂ emissions other than combustion
0.02 – 0.05 kg CO₂/kg
(side reaction from acid use, emissions from sulfide ore)
- the amount of change to resources such as earth, rocks, and water
Ore mined: 20 – 50 kg
(average content 1 – 3% Cu)
Water consumption: 3 – 5 kg (for flotation process)
- input item name and input amount guideline
Copper ore (sulfide ore): 20 – 50 kg
Limestone: 0.2 – 0.5 kg (for pH adjustment)
Sulfuric acid: 0.05 – 0.1 kg (impurities removed)
- process substances such as solvents
Flotation chemicals (e.g. xylene chemicals): 0.005 – 0.01 kg
Process water: 3 – 5 kg (circulation rate: 80 – 90%)
- waste requiring special treatment
Tailings (residue after flotation): 19 – 48 kg
Wastewater (containing impurities): 0.5 – 1 kg
- catalysts and their degradation rates
Degradation of flotation catalyst and chemicals: 0.001 – 0.002 kg/kg
Process Overview
Copper concentrate production relies primarily on flotation from copper ore. With an average ore content of 1-3%, 1 kg of concentrate is obtained from approximately 20-50 kg of ore. After mining, the ore is crushed and the copper is separated using flotation chemicals and water. The flotation process generates tailings and wastewater due to the use of chemicals and requires proper management.
The process involves significant use of electricity and diesel fuel, and because of the heavy use of water resources, it is important to have a circulation system in place.
Nickel concentrate
- input power
3 – 7 kWh/kg
(mining, crushing, flotation or smelting processes)
- input fuel
Diesel fuel: 0.2 – 0.4 kg
(for mining and transportation)
Heavy oil: 0.1 – 0.3 kg (firing furnace)
3 CO₂ emissions other than combustion
0.05 – 0.1 kg CO₂/kg
(generated in sulfuric acid processing and ore reactions)
- the amount of change to resources such as earth, rocks, and water
Ore mined: 40 – 100 kg (average content 1 – 2.5% Ni)
Water consumption: 2 – 4 kg (flotation and refining processes)
- input item name and input amount guideline
Nickel ore (sulfide ore or oxide ore): 40 – 100 kg
Lime: 0.5 – 1.0 kg (for pH adjustment and removal of impurities)
Sulfuric acid: 0.1 – 0.2 kg (leaching process during smelting)
- process substances such as solvents
Extraction solvent (kerosene solvent): 0.01 – 0.05 kg
Process water: 2 – 4 kg (circulation rate: 85 – 90%)
- waste requiring special treatment
Tailings (residue after flotation): 39 – 98 kg
Slag (smelting by-product): 1 – 2 kg
- catalysts and their degradation rates
Leached catalyst (sulfate-based catalyst): Degradation rate 0.002 – 0.005 kg/kg
Process Overview
Nickel concentrates are mainly produced from sulfide or oxide ores. Sulfide ores are crushed and then flotation is performed, while oxide ores are smelted by sulfuric acid leaching. Since the nickel content of the ore is between 1 and 2.5%, a large amount of ore must be processed, which requires a lot of energy for mining and refining.
Management of solvents and acids used for flotation and leaching, as well as disposal of large amounts of tailings, presents environmental challenges. Water circulation is recommended for the process, and catalyst degradation must be minimized.
Ammonium hydroxide (NH₄OH)
- input power
0.1 – 0.2 kWh/kg
(pump operation and temperature control)
- input fuel
Natural gas: 0.05 – 0.1 kg
(used in ammonia synthesis)
3 CO₂ emissions other than combustion
0.01 – 0.02 kg CO₂/kg
(byproduct of ammonia production)
- the amount of change to resources such as earth, rocks, and water
Water usage: 3 – 4 kg
(for process water and dilution)
- input item name and input amount guideline
Ammonia (NH₃): 0.18 – 0.2 kg
(diluted to produce ammonium hydroxide)
Water (H₂O): 0.8 – 1.0 kg
- process substances such as solvents
Cooling water: 2 – 3 kg (circulation 90 – 95%)
- waste requiring special treatment
Spent solvent or cooling water: 0.01 – 0.02 kg (wastewater treatment)
- catalysts and their degradation rates
Degradation rate: 0.0005 – 0.001 kg/kg (ammonia production stage)
Process Overview
Ammonium hydroxide is produced by the reaction of water and ammonia. Ammonia is synthesized mainly from natural gas using the Haber-Bosch process, and ammonium hydroxide is produced by dissolving the produced ammonia in water.
In the production process, temperature and pressure control are critical, and electricity is used primarily for pumps and cooling systems. In addition, iron-based catalysts are used in the synthesis of ammonia, but their degradation rate is so low that frequent catalyst replacement is not necessary.
Because large amounts of water are required, water recycling is used, and wastewater treatment is part of environmental management.
Iodine-bearing concentrates
- input power
0.5 – 1.0 kWh/kg
(used in pumping, stirring, and filtration processes)
- input fuel
Natural gas: 0.2 – 0.5 kg
(heating and distillation process)
3 CO₂ emissions other than combustion
0.01 – 0.03 kg CO₂/kg
(by-product of reaction of extraction chemicals)
- the amount of change to resources such as earth, rocks, and water
Crude ore input: 10 – 15 kg
(iodine concentration: 0.1 – 0.5%)
Water usage: 5 – 10 kg
- input item name and input amount guideline
Iodine-bearing ores (NaI, Ca(IO₃)₂): 10 – 15 kg
Acids (sulfuric acid, hydrochloric acid): 0.5 – 1.0 kg
- process substances such as solvents
Organic solvent (toluene, pentane, etc.): 0.1 – 0.2 kg (circulation rate: 90%)
Cooling water: 15 – 20 kg (circulation rate: 95%)
- waste requiring special treatment
Wastewater (acidic): 0.5 – 1.0 kg (requires neutralization)
Insoluble sludge: 2 – 3 kg
Process Overview
In the production of iodine-bearing concentrates, crude ores containing iodine are used. The main ores are sodium iodide and calcium iodate, from which iodine is extracted using acid. Heating and distillation are required, and fuel consumption is an important factor.
Cooling water and organic solvents are used in some parts of the process but are reused in a circulation system, and wastewater and waste must be treated. Catalyst degradation rates are not an issue in this process, but acid control and ore purity greatly affect the efficiency of production.
acetamide
- input power
1.5 – 2.0 kWh/kg
(consumption in stirring, heating and separation equipment)
- input fuel
Natural gas: 0.3 – 0.5 kg
(heating reaction and distillation process)
3 CO₂ emissions other than combustion
0.05 – 0.1 kg CO₂/kg
(emissions from by-products and solvent use)
- the amount of change to resources such as earth, rocks, and water
Water usage: 5 – 8 kg
Input of ore related to raw materials: not required if concentrate-derived components are required
- input item name and input amount guideline
Acetic acid: 1.2 – 1.5 kg
Ammonia: 0.4 – 0.6 kg
Sulfuric acid (used to neutralize byproducts): 0.1 – 0.2 kg
- process substances such as solvents
Organic solvents (methanol, ethanol): 0.2 – 0.3 kg (circulation rate: 85%)
Cooling water: 10 – 15 kg (circulation rate: 90%)
- waste requiring special treatment
Sulfate sludge: 0.5 – 1.0 kg
Wastewater (containing ammonia): 1 – 2 kg
Process Overview
Acetamide is produced primarily from acetic acid and ammonia, and amidation proceeds through a heating reaction. The process uses acid to neutralize byproducts and requires sludge disposal. In addition, heating and cooling are frequently required in the process, so fuel and power consumption is an important factor.
Solvents are used as part of the reaction system and are reused through a circulation system, but some wastewater must be treated appropriately. Since no catalyst is used, there is no need to control the degradation rate; however, reaction efficiency and waste reduction are key to cost reduction.
palladium (Pd)
- input power
2.5 – 3.5 kWh/kg
(electrolytic refining, melting and refining equipment)
- input fuel
Natural gas: 0.4 – 0.6 kg
(used for heating in the refining process)
3 CO₂ emissions other than combustion
0.3 – 0.5 kg CO₂/kg
(emissions from by-products of solvent and chemical use)
- the amount of change to resources such as earth, rocks, and water
Water usage: 10 – 15 kg
Input of concentrate from crude ore: 200 – 250 kg
(assuming a palladium content of about 0.5 – 0.7% in concentrate)
- input item name and input amount guideline
Hydrochloric acid: 2 – 3 kg
Nitric acid: 1.5 – 2.0 kg
(used for dissolution reaction in royal water)
Sodium sulfite: 0.1 – 0.2 kg (reducing agent)
- process substances such as solvents
Organic solvent (e.g. tributyl phosphorus): 0.1 – 0.2 kg (circulation: 90%)
Cooling water: 20 – 25 kg (circulation rate: 95%)
- waste requiring special treatment
Heavy metal containing sludge: 1 – 2 kg
Acidic liquid waste: 5 – 8 kg
Process Overview
Palladium is produced through a chemical refining and reduction process using concentrates obtained from electrolytic refining by-products of nickel and copper as a starting point. Royal water is used to dissolve palladium, which is then reduced to yield palladium metal. The refining process uses large quantities of acids such as hydrochloric acid and nitric acid, so the treatment of acidic effluents is important.
Solvents are typically used in the process at high recirculation rates, but catalyst degradation must also be managed. Catalyst degradation rates of **0.1 – 0.5%** per kg can be used for extended periods of time.
Aluminum chloride (AlCl₃)
- input power
2.0 – 3.0 kWh/kg
(energy for refining, furnace, etc.)
- input fuel
Natural gas: 0.5 – 0.7 kg
(used for chlorination process at high temperatures)
3 CO₂ emissions other than combustion
0.2 – 0.3 kg CO₂/kg
(emissions during reaction process and acid neutralization)
- the amount of change to resources such as earth, rocks, and water
Water usage: 8 – 12 kg
(cooling and cleaning applications)
Concentrate input: 4 – 6 kg
(as bauxite concentrate)
- input item name and input amount guideline
Aluminum concentrate (aluminum oxide, Al₂O₃): 2 – 3 kg
Chlorine gas: 2 – 2.5 kg
(reacts with aluminum oxide at high temperature to form AlCl₃)
Hydrochloric acid: 0.5 – 0.8 kg (for cleaning and treatment of residual aluminum)
- process substances such as solvents
Cooling water: 15 – 20 kg (circulation rate: 95%)
- waste requiring special treatment
Acidic liquid waste: 3 – 5 kg
Sludge with impurities: 0.5 – 1 kg
- catalyst usage and degradation rate
Catalyst (e.g. iron chloride FeCl₃, if used): 0.01 – 0.02 kg
Degradation rate: 0.1 – 0.5% / kg AlCl₃
Process Overview
Aluminum chloride is formed by the reaction of aluminum oxide (Al₂O₃) with chlorine gas under high temperature conditions. Aluminum oxide from bauxite is used, which requires pre-processing of the ore. The process uses large amounts of chlorine and requires reactions at high temperatures, which involves the consumption of natural gas as fuel. Acidic effluents generated by the process must be treated, and cooling water is typically reused.
Plant 1kg cultivation
- input power
0.1 – 0.5 kWh/kg
(energy for irrigation systems, greenhouses, lighting, etc.)
- input fuel
Diesel oil or natural gas: 0.02 – 0.1 kg
(fuel use in greenhouses and heating facilities)
3 CO₂ emissions other than combustion
0.1 – 0.3 kg CO₂/kg
(due to respiration from soil and decomposition of organic fertilizers)
- the amount of change to resources such as earth, rocks, and water
Water consumption: 300 – 500 kg
(for irrigation, reuse if water circulation system is present)
- input item name and input amount guideline
Nitrogen fertilizer (urea, ammonium sulfate, etc.): 0.01 – 0.02 kg
Phosphate fertilizer (dicalcium phosphate): 0.005 – 0.01 kg
Potash fertilizer (e.g. potassium chloride): 0.005 – 0.01 kg
Pesticides (herbicides, insecticides, fungicides): 0.001 – 0.002 kg
- process substances such as solvents
Irrigation water: 300 – 500 kg (circulation rate: 50 – 80%)
- waste requiring special treatment
Plant residues (e.g. prunings): 0.05 – 0.1 kg
Fertilizer-derived wastewater: 0.05 – 0.1 kg
- catalyst usage and degradation rate (if applicable)
Catalyst (to promote the effect of mycorrhizal fungi and microbial materials): 0.001 – 0.005 kg
Degradation rate: 10 – 20% / kg plant (effect decreases with time)
Process Overview
Fertilizers, mainly nitrogen, phosphorus, and potassium, are applied to grow plants, and irrigation consumes large amounts of water. Greenhouse cultivation increases the use of electricity and fuel. Pesticides are used as needed for plant growth, but may be kept to a minimum. In addition, plant residues and fertilizer-derived wastewater are generated as waste. Instead of catalysts, microbial materials are used to promote plant growth, which require periodic replenishment due to their high rate of degradation.
phosphate
- input power
0.8 – 1.5 kWh/kg
(concentrate processing, heating and stirring of chemical reactions, etc.)
- input fuel
Natural gas or coal: 0.2 – 0.3 kg/kg
(for use in furnaces)
3 CO₂ emissions other than combustion
0.1 – 0.3 kg CO₂/kg
(chemical reaction, by-product of phosphate impurity removal)
- the amount of change to resources such as earth, rocks, and water
Water consumption: 5 – 10 kg/kg
(dissolution, cooling, refining process)
- input item name and input amount guideline
Phosphate concentrate: 1.5 – 2.5 kg
(depending on calcium phosphate concentration in concentrate)
Sulfuric acid: 0.6 – 0.9 kg
(used in the production of phosphoric acid)
- process substances such as solvents
Water: 5 – 10 kg (circulation rate: 70 – 80%)
- waste requiring special treatment
Gypsum waste (calcium phosphate by-product): 1.5 – 2.0 kg
(to be recycled or disposed)
- catalyst usage and degradation rate (if applicable)
Catalyst used (used in small quantities to accelerate acid treatment): 0.001 – 0.005 kg
Degradation rate: 10 – 20% / kg phosphate
Process Overview
To produce phosphate, phosphate concentrate is treated with sulfuric acid to extract phosphoric acid, which is then neutralized to produce phosphate. A large amount of gypsum by-products are generated during the process, which must be disposed of or reused as cement material. Water is circulated through the process, but some is lost through evaporation or as wastewater.
disinfectant
- input power
2 – 4 kWh/kg
(used for reaction temperature control, stirring, and purification)
- input fuel
Natural gas: 0.1 – 0.2 kg/kg
(used for heat supply and heating processes)
3 CO₂ emissions other than combustion
0.05 – 0.1 kg CO₂/kg
(emissions from raw material synthesis and side reactions)
- the amount of change to resources such as earth, rocks, and water
Water consumption: 10 – 15 kg/kg
(for reaction and cooling)
- input item name and input amount guideline
Glutaraldehyde: 0.2 – 0.4 kg
Copper sulfate (CuSO₄): 0.3 – 0.5 kg
Benzalkonium chloride (BKC): 0.1 – 0.3 kg
- process substances such as solvents
Ethanol: 1.0 – 2.0 kg (circulation: 70 – 85%)
- waste requiring special treatment
Containing liquid waste (unreacted material, solvent residue): 1 – 2 kg
(must be treated in an appropriate liquid waste treatment facility)
- catalyst usage and degradation rate (if applicable)
Phosphoric acid catalyst: 0.02 – 0.05 kg
Degradation rate: 15 – 25% / kg fungicide
Process Overview
Glutaraldehyde (organic disinfectant), copper sulfate (inorganic disinfectant component), and benzalkonium chloride (BKC) (disinfectant with surfactant) are typically used to produce disinfectants. These compounds are dispersed in solvents (such as ethanol) at specific concentrations and synthesized or mixed by temperature and agitation control. Cooling water and electricity are heavily used at each stage of the process, and residual effluent must be treated after production. In addition, phosphoric acid-based catalysts are often used, resulting in constant degradation.
manganese concentrate
- input power
1.5 – 2.5 kWh/kg
(operation of ore crushing and beneficiation processes)
- input fuel
Light or heavy oil: 0.05 – 0.15 kg/kg
(used for operating mining and transportation machinery)
3 CO₂ emissions other than combustion
0.03 – 0.05 kg CO₂/kg
(emissions as by-product of chemical processing)
- the amount of change to resources such as earth, rocks, and water
Mining Crude Ore: 6 – 12 kg/kg concentrate
Manganese content of mined ore is 8 – 25
Water consumption: 5 – 10 kg/kg
(used for flotation beneficiation and washing processes)
- input item name and input amount guideline
Mining crude ore (manganese ore): 6 – 12 kg
Flocculant (polymer-based): 0.01 – 0.03 kg
(used in ore beneficiation process)
- process substances such as solvents
Water: 5 – 10 kg (circulation rate: 80 – 90%)
- waste requiring special treatment
Tailings (unprocessed ore, flotation): 5 – 10 kg/kg concentrate
(disposed of or reused in appropriate processing plant)
Process Overview
In the production of manganese concentrates, flotation and gravity beneficiation are used to concentrate the manganese content from the ore. The manganese content of mined ores ranges from 8 – 25%, and a large amount of coarse ore must be processed to obtain concentrates. During the concentrating process, chemicals such as flocculants are used to help separate the ore particles.
The beneficiation process also requires a lot of water, but about 80 – 90% is recycled. Tailings (the residue after beneficiation) must be properly managed. Overall energy use and fuel input depend on the size of the mining operation and the geographical conditions of the mine.
Decanoic acid (C₁₀H₂₀O₂)
- input power
0.8 – 1.5 kWh/kg
(purification, reaction temperature control, mixer operation)
- input fuel
Natural gas: 0.2 – 0.4 kg/kg
(for heating reaction)
3 CO₂ emissions other than combustion
0.05 – 0.1 kg CO₂/kg
(byproduct of chemical reaction)
- the amount of change to resources such as earth, rocks, and water
Water consumption: 8 – 12 kg/kg
(for rinsing and cooling)
- input item name and input amount guideline
Fatty acids from coconut or palm oil: 1.2 – 1.5 kg
Catalyst (acidic catalyst: paratoluenesulfonic acid): 0.01 – 0.05 kg (degradation rate: 1-2%/kg)
- process substances such as solvents
Hexane: 0.5 – 0.8 kg (circulation: 90 – 95%)
- waste requiring special treatment
Impurity residue: 0.1 – 0.2 kg/kg (reprocessed or discarded)
Process Overview
Decanoic acid (capric acid) is obtained by decomposing and refining medium-chain fatty acids found mainly in palm oil and coconut oil. The reaction utilizes a heating reaction and an acidic catalyst, and decanoic acid is purified from the decomposed product by distillation.
Organic solvents such as hexane are used in the reaction system to facilitate separation of the fatty acids, which are often reused. The refining process requires electricity and water. In addition, waste oil and reaction residues are generated as by-products, which require appropriate treatment.
Octanol (C₈H₁₇OH)
- input power
1.5 – 2.0 kWh/kg
(distillation, reaction temperature control, mixing facilities)
- input fuel
Natural gas: 0.3 – 0.6 kg/kg
(fuel for heating, operating reactors and distillation units)
3 CO₂ emissions other than combustion
0.1 – 0.2 kg CO₂/kg
(CO₂ as chemical reaction byproduct)
- the amount of change to resources such as earth, rocks, and water
Water consumption: 10 – 15 kg/kg
(for cooling water and cleaning)
- input item name and input amount guideline
Ethylene: 0.3 – 0.4 kg
Synthesis gas (H₂ + CO): 1.0 – 1.2 kg
Catalyst (e.g. ruthenium catalyst): 0.01 – 0.02 kg (degradation rate: 1% / kg)
- process substances such as solvents
Heptane: 0.5 – 0.7 kg (circulation: 90 – 95%)
- waste requiring special treatment
Reaction residue: 0.1 – 0.2 kg/kg (for reuse or disposal)
Process Overview
The synthesis gas method or the hydrogenation of aldehydes is commonly used to produce octanol. Octanol is obtained by the reduction of aldehydes produced from synthesis gas (H₂ + CO) and ethylene using ruthenium or cobalt-based catalysts. During the production process, solvents such as heptane are used to homogenize the reaction environment and separate the products, ensuring resource efficiency by increasing the circulation rate.
For the heating reaction, natural gas fuel is consumed and large amounts of cooling water are used. The residues produced must be properly disposed of, but some of the byproducts may be reused.
Tributyl phosphorus (TBP)
- input power
0.8 – 1.2 kWh/kg
(reaction temperature control, agitation, purification process)
- input fuel
Natural gas: 0.3 – 0.5 kg/kg
(for heating)
3 CO₂ emissions other than combustion
0.05 – 0.1 kg CO₂/kg
(byproduct of reaction)
- the amount of change to resources such as earth, rocks, and water
Water consumption: 5 – 8 kg/kg
(for reaction, washing, cooling)
- input item name and input amount guideline
Phosphoric acid (H₃PO₄): 0.3 – 0.5 kg
Butanol: 1.2 – 1.5 kg
Sulfuric acid (used as catalyst): 0.05 – 0.1 kg
- process substances such as solvents
Toluene: 0.2 – 0.3 kg (circulation: 90%)
- catalyst use and degradation rate
Sulfuric acid (catalyst): Degradation rate 5 – 10%.
- waste requiring special treatment
Spent sulfuric acid: 0.05 – 0.1 kg/kg
Impurity residue: 0.1 – 0.15 kg/kg
Process Overview
TBP (tributyl phosphate) is produced by the esterification reaction of phosphoric acid (H₃PO₄) with butanol. Sulfuric acid is generally used as a catalyst for the reaction. The resulting TBP is distilled and purified after the reaction. Toluene is often used as a solvent and is circulated.
The process generates spent sulfuric acid and other waste products, so waste acid treatment is required. Energy costs are relatively low, and natural gas is used as the primary fuel.
helium gas
- input power
15-20 kWh/kg
Helium separation and liquefaction processes require compression, cooling, and distillation, which are highly power intensive.
- input fuel
Natural gas: 250-300 kg
(average helium content in natural gas is 0.3-0.5%)
- non combustion CO
0.2 to 0.3 kg
Secondary emissions generated by some separation processes.
- the amount of change in resources such as earth, rocks, and water
Water: 10-20 L/kg
Consumed as refrigerant in gas cooling and liquefaction processes.
- input
Natural gas: 250-300 kg
Helium is contained in trace amounts in natural gas, and in some cases residual gas is reused after extraction.
- process substances such as solvents
Liquid nitrogen: 10-15 kg
Used for low-temperature separation processes.
Circulation rate: 50-70
- catalyst use and degradation rate
No specific catalyst is required.
- waste requiring special treatment
Carbon dioxide/noncombustible gas: 2-3 kg
Formed as a byproduct after natural gas processing.
supplementary information
Helium extraction is centered on gas separation from natural gas and liquefaction at very low temperatures. Since the concentration of helium in natural gas is very low, large amounts of natural gas must be processed. In addition, obtaining liquid helium requires a more sophisticated cooling process, which consumes a large amount of energy.
The following is a rough estimate of the energy and inputs required to produce 1 kg of liquid nitrogen. Liquid nitrogen is produced in an air separation unit (ASU: Air Separation Unit), which requires a lot of energy for cooling and liquefaction.
liquid nitrogen
- input power
0.6 to 1 kWh/kg
Electricity is consumed to compress, cool, separate, and liquefy air.
- input fuel
Natural gas: 0.02-0.05 kg
May be used as an energy source for power supply in the air separation process.
- non combustion CO
Almost none (only indirect emissions)
- the amount of change to resources such as earth, rocks, and water
Water: 2-5 L/kg
Used as circulating water for cooling.
- input
Air: 833 kg
(Nitrogen ratio in air is about 78%, about 833 kg of air is used to obtain 1 kg of liquid nitrogen)
- process substances such as solvents
Refrigerant gas (e.g., argon): small quantity (circulation usage: 90% or more)
- catalyst use and degradation rate
No specific catalyst is used.
- waste requiring special treatment
Carbon dioxide: 0.01 to 0.05 kg
Some CO₂ in the air may be condensed.
supplementary information
Liquid nitrogen is produced by the low-temperature separation method, which separates the components of air (oxygen, nitrogen, etc.) using a temperature difference. Since a large amount of air is compressed and the nitrogen component is separated and liquefied while cooling, extremely efficient compression equipment is required. The largest cost factor in manufacturing is electricity, and the supply of electricity using renewable energy is the key to reducing CO₂.
neon (Ne)
Neon is a rare gas that is separated and recovered from air and is produced using an air separation unit (ASU).
- input power
50-60 kWh/kg
Neon is only about 0.0018% in the atmosphere and must be purified by compressing and cooling large volumes of air.
- input fuel
Natural gas: 0.1 to 0.3 kg
when used to supply power for air separation processes.
- non combustion CO
0 kg
Neon production itself has no direct CO2 emissions.
- the amount of change to resources such as earth, rocks, and water
Water: 5-10 L/kg
Used for air cooling and cooling water, but mostly reused.
- input
Air: 555,000 kg
(based on the atmospheric content of neon, this much air is needed to obtain 1 kg of neon)
- process substances such as solvents
Refrigerant (e.g., argon or nitrogen): 0.01 kg (circulation rate > 90%)
- catalyst use and degradation rate
No catalyst (no catalyst is used in neon separation)
- waste requiring special treatment
Carbon dioxide (CO₂): 0.05-0.1 kg
May be emitted in the compression process when recovered from the atmosphere.
supplementary information
Neon recovery, like other noble gases, takes place in very large air separation units and requires enormous amounts of air for purification. During the air separation process, oxygen and nitrogen are first extracted, and finally trace amounts of neon are separated. Due to its high energy cost, recovered neon is mainly used in industrial applications (e.g., semiconductor manufacturing and lasers).
argon (Ar)
Argon is a noble gas that makes up about 0.93% of air and is primarily recovered by air separation units (ASUs).
- input power
5-8 kWh/kg
Electric power for compressing and cooling air and separating it from oxygen and nitrogen.
- input fuel
Natural gas: 0.2-0.5 kg
Used as an energy source in some air separation facilities.
- non combustion CO
0.05 to 0.1 kg
CO2 emitted during compression or indirectly from exhaust.
- the amount of change to resources such as earth, rocks, and water
Water: 5-15 L/kg
Used as cooling water, some of which is reused.
- input materials Name and input amount
Air: 12,000 to 15,000 kg
Large volumes of air are required due to the presence of 0.93% argon in the atmosphere.
- process substances such as solvents
Refrigerant (e.g., liquid nitrogen): 0.01 to 0.02 kg (circulation rate >90%)
- catalyst use and degradation rate
No catalyst (argon separation does not require a catalyst)
- waste requiring special treatment
Oxygen and nitrogen gas: dozens of kilograms (used in other processes, some discharged)
supplementary information
Argon is widely used in the steel, semiconductor, and chemical industries. The air separation process is very energy intensive and requires efficient facility operation. As a byproduct of argon production, oxygen and nitrogen are also recovered and utilized in other industrial applications.
Krypton (Kr)
Krypton is found in only a very small amount of air (about 0.0001%) and is commonly produced in air separation units (ASUs).
- input power
15-25 kWh/kg
Electricity required to compress, cool, and separate air.
- input fuel
Natural gas: 0.3-0.5 kg
Used as an energy source in some facilities.
CO2 emissions other than combustion
0.1 to 0.2 kg
CO2 emitted indirectly in the compression and separation processes.
- the amount of change to resources such as earth, rocks, and water
Water: 10-20 L/kg
Water use for cooling and cleaning.
- name of input and input amount
Air: approx. 10,000-15,000 kg
A large amount of air is required for krypton extraction (0.0001% air content).
- process substances such as solvents
Refrigerant (e.g. liquid nitrogen): 0.01 to 0.03 kg (circulation rate >90%)
Used as a cooling medium in the separation process.
- catalyst use and degradation rate
No catalyst required (no catalyst is used for krypton separation)
- waste requiring special treatment
Excess nitrogen and oxygen gas: several tens of kilograms (can be reused for other purposes, but some is discharged)
supplement
Krypton is used in lighting (fluorescent lamps), aerospace, and laser technology. The production process is energy intensive and is obtained as a byproduct along with other gases such as oxygen and nitrogen. Krypton production is basically the same as for other noble gases (argon and neon), where air is cooled and separated into its component parts.
Xenon (Xe)
Xenon is extracted with other gases such as oxygen and nitrogen in the air separation unit (ASU) because its concentration in the atmosphere is very low.
- input power
30-50 kWh/kg
Electricity required to compress, cool, and separate air.
- input fuel
Natural gas: 0.4-0.6 kg
Used for energy supply in some plants.
CO2 emissions other than combustion
0.2 to 0.3 kg
Indirect emissions from the compression process and cooling system.
- the amount of change to resources such as earth, rocks, and water
Water: 15-30 L/kg
Used for cooling and cleaning applications.
- name of input and input amount
Air: 15,000-20,000 kg
Xenon is extremely rare, with an air content of only 0.0000087%, so very large quantities of air are required.
- process substances such as solvents
Refrigerant (liquid nitrogen): 0.02-0.05 kg (circulation rate of 90% or more)
Used for cooling in the separation process.
- catalyst use and degradation rate
No catalyst required (no catalyst is used for xenon separation)
- waste requiring special treatment
Oxygen and nitrogen surplus gas: 100-200 kg
Some will be reused for other industrial applications, but surpluses may occur.
supplement
Xenon is used in a wide range of applications, including lighting (automobile headlights and projectors), space exploration (ion propulsion), and medical gases. Extraction by air separation is energy intensive and expensive to produce, but since it is obtained as a byproduct of other gases such as oxygen and nitrogen, overall production efficiency is a consideration.
radon (Rn)
Radon (Rn) is a noble gas, but it is very rare in nature and has very limited industrial production and commercial applications. The following is a rough estimate of inputs and energy to show hypothetical production of 1 kg of radon. However, commercial production of radon is very specific and is almost always obtained through radioactive decay (emissions from uranium ore or radium).
- input power
30-60 kWh/kg
Used in air separation units and concentration processes.
- input fuel
Natural gas: 0.4-0.6 kg
Used in some cooling and separation processes.
- non combustion CO
0.1 to 0.3 kg
Indirect emissions from compression and cooling systems.
- the amount of change to resources such as earth, rocks, and water
Ore (including water): thousands of kg of ore are needed
To obtain trace amounts of radon from uranium ore, the ore must be processed.
- input item name and input amount guideline
Uranium ore (or radium-bearing material): 1,000-3,000 kg
Radon is obtained during the decay process of radium-226.
- process substances such as solvents
Refrigerant (liquid nitrogen): 0.01-0.02 kg (95% circulation rate)
Used for rare gas separation.
- catalyst use and degradation rate
No catalyst required
No catalyst is used because it is a radioactive decay process.
- waste requiring special treatment
Radioactive waste: 100-200 kg
Radioactive material remaining after ore processing needs to be processed.
supplement
The main sources of radon are radioactive decay of uranium ore and radium. It is not produced on a large commercial scale and is usually limited to research applications or as a medical gas. The handling of radioactive materials is strictly regulated and waste disposal requires a high degree of control.
Bromine (Br₂)
Bromine is usually concentrated from salt lake water or seawater and extracted by chemical processes.
- input power
2-5 kWh/kg
Used for electrolysis, evaporative concentration and pumping systems.
- input fuel
Natural gas: 0.2-0.5 kg
Used as a heat source to heat salt lake water.
- other than combustion CO
0.1-0.2 kg CO₂
Indirect emissions in the process (upstream processes such as electricity generation).
- the amount of change to resources such as earth, rocks, and water
Salt
lake water: 10,000-30,000 kg
Bromine concentration is usually around 0.05%, requiring large quantities of salt lake water.
- input item name and input amount guideline
Sodium chloride: 1 to 2 kg
Aids in the electrolysis process. May be required for bromine enrichment.
- process substances such as solvents
Hydrochloric acid: 0.1-0.5 kg (circulation rate 80-90%)
Used for bromine redox reaction.
- catalyst use and degradation rate
No
catalyst required No catalyst required as it is produced by electrolysis and oxidation reactions.
- waste requiring special treatment
Effluent (high salt content): 5-10 kg
Effluent remaining after the salt lake water concentration process.
Process Overview
Bromine is typically recovered from salt lake water. Water containing high concentrations of bromine is converted to bromine gas through evaporation and oxidation processes, which is then cooled to a liquid. Electricity and fuel are used for heating, cooling, and evaporation. Hydrochloric acid may also be used as an oxidizing agent. Waste management is required because the extraction and treatment of salt lake water produces wastewater.
Iodine (I₂)
Iodine is recovered primarily from groundwater and seawater brines.
- input power
2-5 kWh/kg
Used for pumping systems, evaporation and cooling operations.
- input fuel
Natural gas: 0.3-0.6 kg
Used as heat source during iodine extraction.
- other than combustion CO
0.2-0.3 kg CO₂
Indirect emissions throughout the process (upstream of electricity consumption and heat use).
- the amount of change to resources such as earth, rocks, and water
Groundwater or salt water: 15,000 to 25,000 kg
Large volumes of water sources with low iodine content need to be treated.
- input item name and input amount guideline
Oxidizer (sulfuric acid or chlorine): 1 to 2 kg
Used to convert iodide to iodine.
- process substances such as solvents
Sulfuric acid: 0.5-1.0 kg (90% recirculation
) Used for oxidation reactions and often reused.
- catalyst use and degradation rate
No catalyst required No catalyst is used because it is produced by a chemical reaction using an oxidizing agent.
- waste requiring special treatment
Highly concentrated salt wastewater: 10-20 kg
Concentrated wastewater after treatment. Treatment is required according to environmental standards.
Process Overview
Iodine is produced mainly by the process of oxidizing iodide recovered from groundwater or seawater and converting it to pure iodine. It is purified through distillation and crystallization to yield iodine crystals as the final product. This production requires electricity for pumping systems and natural gas for heating, and involves a large amount of water treatment.
While production efficiency and environmental impact depend on raw material supply and plant operating conditions, wastewater treatment and reuse systems are key issues.
Salt water (salt concentrated water)
- input power
0.005-0.01 kWh/kg
Used as energy for seawater intake pumps and membrane filtration.
- input fuel
Natural gas: 0.02-0.05 kg
Used in thermal treatment processes (when using multi-stage flash evaporation).
- other than combustion CO
0.01 to 0.03 kg CO₂
Emissions from electricity consumption and upstream energy production.
- the amount of change to resources such as earth, rocks, and water
Seawater: approx. 10-15 kg
Amount of seawater processed to obtain 1 kg of brine.
- input item name and input amount guideline
Antifoulant (e.g., sodium hypochlorite): 0.001-0.002 kg
Used to prevent contamination of piping and membranes.
- process substances such as solvents
Membrane cleaning agent (acidic or alkaline): 0.0005-0.001 kg (circulation rate 90%)
For periodic maintenance of membrane
filtration equipment.
- catalyst use and degradation rate
No catalyst required
- waste requiring special treatment
Concentrated seawater (high salinity): 1.2 to 1.5 kg
Returned to the sea, but must be managed in consideration of environmental impact.
Process Overview
Brine is obtained by concentrating seawater through filtration or evaporation. Membrane filtration (RO: reverse osmosis) and multi-stage flash evaporation methods are mainly used. Electricity is consumed when the seawater is drawn in by intake pumps and passed through filtration equipment, and natural gas is used as a heat source in the evaporation method. Treatment and disposal of the concentrated brine is important to reduce environmental impact.
The efficiency of the manufacturing process depends on the equipment and conditions, but electricity and heat sources are the primary energy components.
Sodium Lauryl Sulfate (SLS)
- input power
1.5 to 2.0 kWh/kg
Used to operate stirring, reaction, and refining equipment throughout the process.
- input fuel
Natural gas: 0.1-0.2 kg
May be used in the heating process (sulfation process).
CO₂ emissions other than combustion
0.05-0.1 kg CO₂/kg
Emissions from chemical reactions and indirect electricity consumption throughout the process.
- the amount of change to resources such as earth, rocks, and water
Water: 5-10 kg
Used in reaction and cleaning processes.
- input item name and input amount guideline
Lauryl alcohol (C12 alcohol): 0.8-0.9 kg
Lauryl alcohol is obtained from coconut oil or palm kernel oil.
Trioxide sulfate (SO₃): 0.3-0.4 kg
Used for sulfation of lauryl alcohol.
- process substances such as solvents
Water: 5-10 kg (approx. 90% circulation)
Used for cleaning and dilution.
- catalyst use and degradation rate
No catalyst required Sulfation
reaction proceeds without a catalyst.
- waste requiring special treatment
Effluent (contained organics and sulfate): 0.5 to 1 kg
treatment is required and appropriate effluent management is required.
Process Overview
Sodium lauryl sulfate is produced primarily by the reaction of **lauryl alcohol with trioxide sulfate (SO₃)**. In this process, lauryl alcohol is sulfated to produce lauryl sulfate, which is further neutralized with sodium hydroxide to yield SLS. The reaction proceeds at high temperatures, so energy input is required, and natural gas or electricity is used for heating.
Disposal of liquid waste is important, and management is essential considering the environmental impact of the byproducts generated.
EDTA (ethylenediaminetetraacetic acid)
- input power
2.5 to 3.0 kWh/kg
For use in stirring
, reaction, drying processes, etc.
- input fuel
Natural gas: 0.1-0.2 kg
Used for heating process.
CO₂ emissions other than combustion
0.05-0.1 kg CO₂/kg
Byproduct of chemical reaction of raw materials.
- the amount of change to resources such as earth, rocks, and water
Water: 10-20 kg
Used for washing and reaction.
- input item name and input amount guideline
Ethylenediamine: 0.6-0.7 kg
Raw material providing the basic structure of chelating agents.
Acetic acid (or sodium acetate): 0.5-0.6 kg
Used as a source of acetic acid base.
Formaldehyde: 0.3-0.4 kg
Reacts with ethylenediamine to introduce carboxy groups.
- process substances such as solvents
Water: 10-20 kg (90% circulation)
Used for reaction and purification of products.
- catalyst use and degradation rate
Catalysts are usually not required. However, acids and alkalis may be used as reaction accelerators.
- waste requiring special treatment
Organic waste liquid: 0.5 to 1.0 kg
Waste containing unreacted raw materials and
byproducts is generated and must be treated.
Process Overview
EDTA is produced by the condensation reaction of ethylenediamine, acetic acid or acetate, and formaldehyde. Typically, these reactions are carried out at high temperatures in aqueous solution, and the final product is conditioned with an acid or base, dried, and commercialized as powdered EDTA.
Waste management is important, and unreacted materials and byproducts must be treated in such a way that they do not impact the environment.
Lauryl alcohol (dodecanol)
- input power
2-4 kWh/kg
Used in distillation and heating processes.
- input fuel
Natural gas: 0.1-0.3 kg
Used as a heating source for distillation and refining.
CO₂ emissions other than combustion
0.05-0.1 kg CO₂/kg
Byproducts from raw materials and reaction processes.
- the amount of change to resources such as earth, rocks, and water
Water: 15-30 kg
Used for reaction and cooling.
- input item name and input amount guideline
Coconut or palm kernel oil: 1.2 to 1.5 kg
Natural source of fatty acids as main ingredient.
Hydrogen: 0.05-0.1 kg
Important raw material for hydrolysis and hydrogenation.
- process substances such as solvents
Methanol: 0.5-1.0 kg (90% circulation)
Used for methyl esterification reaction of fatty acids.
- catalyst use and degradation rate
Copper or nickel-based catalyst: 0.01-0.05 kg (degradation rate: 1-2%/kg
) Used to accelerate the hydrogenation reaction.
- waste requiring special treatment
Waste liquids (including unreacted materials and byproducts): 0.3-0.5 kg
Waste generated from the refining process.
Process Overview
Lauryl alcohol is produced industrially by hydrogenation of natural fatty acids. Generally, coconut oil or palm kernel oil is used as a raw material to produce fatty acid esters, which are then hydrogenated to obtain lauryl alcohol. In the process, nickel-based catalysts are used in most reactions, and methanol is circulated.
Hydrogen supply is a key element in this production process, and the treatment of byproducts and effluents is also an important issue for reducing environmental impact.
Trioxide sulfate (SO₃)
- input power
0.1-0.2 kWh/kg
Used for compressed air supply and process control.
- input fuel
Natural gas: 0.15-0.25 kg
Provides the thermal energy required by the combustion furnace.
CO₂ emissions other than combustion
0.02-0.05 kg CO₂/kg
By-product of raw gas.
- the amount of change to resources such as earth, rocks, and water
Water: 10-20 kg
SO₃ used in the process of contact with dilute sulfuric acid in a controlled environment after formation.
- input item name and input amount guideline
Sulfur (S): 0.45-0.5 kg
Used for burning to produce sulfur dioxide (SO₂).
Oxygen (O₂): 0.25 to 0.35 kg Oxygen
is supplied to oxidize sulfur dioxide to sulfur trioxide.
- process substances such as solvents
Dilute sulfuric acid: 1 to 2 kg (circulation ratio: 90% or more)
Used in the sulfuric acid production process while being recirculated.
- catalyst use and degradation rate
Vanadium pentaoxide (V₂O₅) Catalyst: 0.01-0.05 kg (degradation rate: 0.5-1%/kg)
Used for SO₂ to SO₃ oxidation reaction.
- waste requiring special treatment
Exhaust gas (including trace amount of unreacted gas): 0.1-0.2 kg
Needs to be purified by an exhaust gas treatment system.
Process Overview
Trioxide sulfate is usually produced by the Contact Process.
Sulfur dioxide (SO₂) is produced by burning sulfur.
SO₂ reacts with oxygen and is converted to sulfur trioxide (SO₃) using a vanadium pentaoxide catalyst.
The production process involves gas circulation and temperature control to increase reaction efficiency. The SO₃ produced is either stored as is or used to react with dilute sulfuric acid to produce sulfuric acid.
From the standpoint of environmental protection, removal of SO₂ and unreacted gases from exhaust gas is important. In addition, the catalysts used deteriorate and must be replaced periodically.
Coconut oil (1kg)
- input power
0.5-0.8 kWh/kg
Used to run crushers, drying facilities, and refining equipment.
- input fuel
Biomass (coconut shells or fiber): 0.3-0.5 kg
Used as a heat source for the drying process.
CO₂ emissions other than combustion
0.05-0.1 kg CO₂/kg
Fermentation by-products and processes.
- the amount of change to resources such as earth, rocks, and water
Water: 10-15 kg
used for coconut washing and process cooling.
- input item name and input amount guideline
Coconut fruit (nut portion): 4-6 kg
Oil extracting material.
- process substances such as solvents
Hexane: 0.05-0.1 kg (recirculation rate >90%
) Used in solvent extraction method in some plants.
- catalyst use and degradation rate
None (no catalyst is used in the standard process)
- waste requiring special treatment
Coconut shells and fiber: 3-4 kg
Some reused for biofuel or compost.
Wastewater: 5-10 kg
Some of the water used in the process requires wastewater treatment.
Manufacturing Process Overview
The production of coconut oil proceeds in the following steps
Harvesting and pulp extraction: Pulp is extracted from the coconut fruit and dried (copra formation).
Pressing or solvent extraction: The dried pulp is pressed or a solvent (hexane) is used to extract the oil.
Refining: Extracted oil is refined to remove impurities and finished as a final product.
During production, some mills increase sustainability by effectively utilizing coconut shells and fiber as biomass fuel. In addition, wastewater and by-products are properly treated and reused.
Dilute sulfuric acid (1kg)
- input power
0.2-0.3 kWh/kg
(used for manufacturing equipment, mixing, pump power, etc.)
- input fuel
Natural gas: 0.05-0.1 kg
(used for heating in the sulfuric acid production process)
3 CO₂ emissions other than combustion
0.01-0.02 kg CO₂/kg
(generated during chemical reactions and from by-products)
- the amount of change to resources such as earth, rocks, and water
Water: 1.5 to 2 kg
(used to dilute the acid)
- input item name and input amount guideline
Concentrated sulfuric acid: 0.3-0.4 kg
(base material for dilute sulfuric acid)
Water: 0.6-0.7 kg
(for acid dilution)
- process substances such as solvents
None (no solvent is used in normal dilute sulfuric acid production)
- catalyst use and degradation rate
None (no catalyst is used in the dilute sulfuric acid production process)
- waste requiring special treatment
Wastewater from dilution process: 0.05-0.1 kg
(requires appropriate neutralization and treatment)
Manufacturing Process Overview
Dilute sulfuric acid is produced primarily by mixing concentrated sulfuric acid with water in the appropriate ratio and adjusting the acid concentration.
Production safety is critical, and a controlled cooling process is essential because heat is generated when mixing water and concentrated sulfuric acid.
The energy used is primarily needed to operate the facility’s pumps and cooling systems.
Fuels such as natural gas may be used for heating processes and equipment maintenance throughout the process.
Dilute sulfuric acid is used in many industries for neutralization, cleaning, or as a raw material for chemical reactions.
V₂O₅ (vanadium pentoxide) catalyst
- input power
0.3-0.5 kWh/kg
(used for crushing, mixing, and heating equipment operation)
- input fuel
Natural gas: 0.15-0.3 kg
(used for heating in the firing process)
3 CO₂ emissions other than combustion
0.02-0.05 kg CO₂/kg
(part of CO₂ byproduct in the process)
- the amount of change to resources such as earth, rocks, and water
Cooling water: 2-4 kg
(used in high-temperature reactors and cooling facilities)
- input item name and input amount guideline
Vanadium concentrate (V₂O₅ content 50%): 1.5-2.0 kg
Sulfuric acid: 0.1-0.2 kg
(for dissolution and purification process)
- process substances such as solvents
Water: 2-3 kg (circulation rate: 80-90%)
- catalyst use and degradation rate
Catalyst degradation rate: 1-2%/year
(when used in sulfur dioxide oxidation process, etc.)
- waste requiring special treatment
Residual waste: 0.1-0.2 kg (e.g., liquid waste containing impurities)
Manufacturing Process Overview
Vanadium concentrate refining:
Separation and refining of vanadium oxides from vanadium-bearing ores.
Oxidation Process:
Vanadium concentrates are calcined and recovered in the form of V₂O₅. Natural gas is commonly used for oxidation.
Catalyst formation:
The resulting V₂O₅ is formed and sintered into a usable catalyst.
This catalyst is particularly important in the production of sulfuric acid (H₂SO₄) from sulfur dioxide (SO₂) and in oxidation reactions of organic compounds.
Sodium metal (Na)
- input power
12-15 kWh/kg
(used for electrolysis of sodium chloride)
- input fuel
Natural gas: 0.2-0.5 kg
(to assist in heating the electrolyzer at high temperatures)
3 CO₂ emissions other than combustion
0.01-0.02 kg CO₂/kg
(from consumption of process by-products and peripheral equipment)
- the amount of change to resources such as earth, rocks, and water
Cooling water: 3-5 kg
(used in the post-reaction sodium cooling process)
- input item name and input amount guideline
Sodium chloride (NaCl concentrate): 1.5-2.0 kg
(sodium is produced by electrolysis)
Calcium salt: 0.1-0.2 kg
(used to control side reactions during electrolysis)
- process substances such as solvents
Electrolyte (salt melt): 0.2-0.5 kg (circulation rate: >90%)
- catalyst use and degradation rate
Graphite electrode: degradation rate 2-5%/year
(used as an electrode, but has a limited service life)
- waste requiring special treatment
Waste brine: 0.5 to 0.8 kg
(requires treatment of non-reusable brine)
Manufacturing Process Overview
Dow Method:
Sodium is obtained primarily by high-temperature fusion electrolysis of sodium chloride (NaCl). In this method, salt is melted at about 600°C and metallic sodium is formed at the cathode.
Byproduct treatment:
Chlorine gas is produced at the anode by the electrolytic reaction, and waste brine is generated as a byproduct.
Cooling and Purification:
Sodium is recovered in a liquid state and cooled to be converted to a solid. Large amounts of water are used to cool the electrolyzer.
This process consumes a lot of energy in a large-scale industrial process, so fuel support and electricity, such as natural gas, are essential. In addition, the byproduct chlorine gas must be treated.
The main composition of the salt melt electrolyte is as follows
Composition of salt fusion electrolyte
Main ingredients: sodium chloride (NaCl)
It is a major component of salt melts and is used to reduce sodium at the cathode.
Melting temperature: approx. 800°C.
Auxiliary ingredients: calcium chloride (CaCl₂) or barium chloride (BaCl₂)
It lowers the melting temperature of NaCl and stabilizes the electrolytic reaction.
NaCl alone melts at about 800°C, but the addition of CaCl₂ or BaCl₂ lowers the temperature to about 600°C.
Lithium chloride (LiCl) or potassium chloride (KCl)
It improves the conductivity of the electrolyte and aids in efficient current supply.
The addition ratio is about 5-10%.
Example of electrolyte ratio
NaCl: 50-70%.
CaCl₂ or BaCl₂: 20-30
LiCl or KCl: 5-10%.
Purpose and Effectiveness of the Process
Mix multiple salts to maintain a stable melt and increase current efficiency.
Lower melting point reduces energy consumption.
Each component can be used on a cyclical basis and requires periodic replenishment.
Electrolytes of this composition are optimized to maximize power efficiency and ensure process stability.
Coconut Fruit
Input power
0.1~0.2 kWh/kg
(used in irrigation pumps and processing facilities)
Input fuel
Diesel oil: 0.02 to 0.04 kg
(fuel for tractors and transport vehicles)
CO₂ emissions other than combustion
0.15 kg CO₂/kg
(indirect emissions from fertilizer production, irrigation systems, etc.)
Amount of change to resources such as soil and water
Water: 800-1000 L/kg
(precipitation or irrigation during growing season)
Input items and their guidelines
Fertilizer (urea, phosphate, potash fertilizer): 0.05-0.1 kg total
Pesticides (insecticides and fungicides): 0.002-0.005 kg
Solvents and other process substances
None (no special solvents are needed for normal coconut fruit cultivation)
Cycloparametric rate
Fertilizers and pesticides: Almost zero (consumed and absorbed into soil)
Waste requiring special treatment
Coconut shell and fiber waste: 0.25-0.3 kg
(Fiber can also be used as a byproduct)
explanation
Coconut cultivation primarily requires water for irrigation and fuel for transportation. Coconut shells and fiber may be discarded, but they are also reusable by-products. In addition, if irrigation is used, the inputs of electricity and water are increased.
Vanadium concentrates
Input power
1.5 to 2.0 kWh/kg
(used in beneficiation and crushing processes)
Input fuel
Diesel oil: 0.05-0.1 kg
(Fuel for heavy machinery and transport vehicles)
CO₂ emissions other than combustion
0.1~0.15 kg CO₂/kg
(indirect emissions from chemical production and machine use)
Amount of change to resources such as soil and water
Ore input: 20-30 kg
(typical vanadium content: 1.5-5%)
Water: 5-10 L/kg
(Used in flotation and other wet processes)
Input items and their guidelines
Dilute sulfuric acid: 0.2-0.4 kg
(Used for leaching process)
Flotation agent: 0.01 to 0.03 kg
(used in the separation process by flotation)
Solvents and other process substances
Organic solvent: 0.05-0.1 kg
(for extraction separation from leachate)
Circulation rate: 60-80
(Solvent recovery after use is possible)
Waste requiring special treatment
Tailings (beneficiation waste): 19-29 kg
(ore residue after beneficiation)
Sulfuric acid waste: 1 to 2 L
(Neutralization treatment required)
explanation
Vanadium concentrates are produced using an acidic leach solution or flotation process. Because of the low vanadium content in the ore, about 20 to 30 kg of raw ore must be processed. Some of the solvents and sulfuric acid used in the process can be reused, but tailings management is important.
Since this process uses mine haulage vehicles and heavy equipment, fuel consumption and indirect CO₂ emissions must also be considered.
Lithium chloride (LiCl)
Input power
5 to 7 kWh/kg
(used in concentration, electrolysis, and drying processes)
Input fuel
Natural gas: 0.1-0.2 kg
(used for enrichment and thermal treatment processes)
CO₂ emissions other than combustion
0.05~0.1 kg CO₂/kg
(indirect emissions from chemical reactions and transportation)
Amount of change to resources such as soil and water
Lithium concentrate: 6-7 kg
(5-6% lithium content from raw ore)
Water: 8 to 10 L
(used for lithium dissolution and filtration)
Input Item Name and Input Quantity Guideline
Sulfuric acid: 1 to 1.5 kg
(for dissolving and neutralizing lithium)
Hydrochloric acid: 0.5 to 1.0 kg
(Accelerates the formation of lithium chloride)
Solvents and other process substances
Organic solvent: 0.05-0.1 kg
* (used in lithium refining process, some times)
Approximate inputs and energy to produce 1 kg of lithium chloride (LiCl)
Input power
4 to 6 kWh/kg
(consumed in the concentration, drying, and product separation processes)
Input fuel
Natural gas: 0.15-0.3 kg
(used in the enrichment process and in the operation of drying furnaces)
CO₂ emissions other than combustion
0.02~0.05 kg CO₂/kg
(emissions during chemical processes and transportation)
Amount of change to resources such as soil and water
Lithium concentrate: 5-7 kg
(assuming a lithium oxide concentration of 5-6% in the lithium ore)
Water: 8-12 L
(used for dissolution and cleaning processes)
Input Item Name and Input Quantity Guideline
Sulfuric acid: 1.2 to 1.5 kg
(for dissolving lithium)
Hydrochloric acid: 0.8 to 1.0 kg
(Used to accelerate chloride formation)
Solvents and other process substances
Organic solvent (e.g., alcohol): 0.05-0.1 kg
(used in some refining processes, some recoverable)
Circulation rate: 80-90%.
Waste requiring special treatment
Sodium sulfate waste: 0.5-0.8 kg
(By-product of lithium extraction process)
Waste acid solution: 0.1-0.2 kg
In this process, lithium extracted from brine or ore is chlorinated to produce lithium chloride through drying and filtration. Energy is consumed primarily in the concentration and drying processes, with sulfuric acid and hydrochloric acid being the key chemical inputs.
Lithium concentrate
Input power
0.5 to 0.8 kWh/kg
(energy consumption in crushing, beneficiation, and concentration processes)
Input fuel
Diesel fuel: 0.02 to 0.05 kg
(used for mining and transportation)
CO₂ emissions other than combustion
0.1-0.2 kg CO₂/kg
(due to mine development and waste disposal)
Amount of change to resources such as soil and water
Raw ore: 5-8 kg
(Assuming lithia pyroxene ore with lithium oxide content of 5-8%)
Water: 3 to 5 L
(consumed during flotation beneficiation and washing processes)
Input Item Name and Input Quantity
Sulfuric acid: 1.0-1.5 kg
(Used to accelerate the extraction of lithium from ore)
Sodium hydroxide: 0.5-0.8 kg
(pH adjustment and precipitation treatment)
Solvents and other process substances
Organic solvent (e.g., alcohol): 0.05 kg
(Used in some separation processes, 90% circulation)
Waste requiring special treatment
Waste acid solution: 0.2-0.5 kg
(generated in the extraction process)
Beneficiation residue: 3-5 kg
(Impurities portion of ore)
In this process, concentrates are obtained from lithium ores such as lithia pyroxene and petalite. Multiple steps are required, including ore crushing, flotation beneficiation, and chemical extraction, and much energy is consumed in crushing and concentration. Sulfuric acid and sodium hydroxide are important inputs in the process, and tailings and waste acid are generated as by-products.
selenium (Se)
Input power
1.5 to 2.0 kWh/kg
(Electricity consumed in electrolytic refining, concentration and refining processes)
Input fuel
Natural gas: 0.3-0.5 kg
(Used as heat source in reduction furnace)
CO₂ emissions other than combustion
0.5 to 1.0 kg CO₂/kg
(emissions from chemical reactions and refining during production)
Amount of change to resources such as soil and water
Copper concentrate residue: 15-20 kg
(Selenium is recovered as a byproduct of the copper refining process)
Water: 2 to 3 L
(used in the cleaning and purification process)
Input Item Name and Input Quantity
Copper anode mud: 5-7 kg
(Refining residue containing selenium)
Sulfuric acid: 0.8 to 1.2 kg
(used for dissolving and separating selenium oxide)
Solvents and other process substances
Organic solvents: 0.05-0.1 kg
(used in sorting and refining processes, circulation rate >90%)
Waste requiring special treatment
Sludge waste: 1 to 2 kg
(Impurities from refining process)
Waste acid solution: 0.3-0.5 kg
(By-product from the dissolution process)
Description.
Selenium is mainly obtained as a by-product of copper refining. Selenium is recovered from the anode mud produced in the copper electrolytic refining process, and then concentrated and purified. Selenium is dissolved in sulfuric acid and oxidized/reduced to obtain pure selenium. The production process consumes a lot of electricity, and natural gas is used as a heat source in the reduction process.
Copper anode mud
Input power
0.2~0.3 kWh/kg
(Electricity consumption in the electrolytic refining process of copper)
Input fuel
Natural gas: 0.1-0.2 kg
(used for furnace heating)
CO₂ emissions other than combustion
0.05~0.1 kg CO₂/kg
(generated in the process of byproduct formation and treatment)
Amount of change to resources such as soil and water
Copper concentrate: 25-30 kg
(Anode mud is obtained as electrolytic refining residue from concentrate)
Water: 3 to 5 L
(used for cleaning and cooling processes)
Input Item Name and Input Quantity Guideline
Copper concentrate: 25-30 kg
(containing approx. 0.2-0.5% gold, silver, selenium, tellurium, etc.)
Sulfuric acid: 0.5 to 1.0 kg
(used as electrolyte)
Solvents and other process substances
Sulfuric acid electrolyte: 5 to 7 L (circulation rate of 90% or more)
(used in electrolytic refining)
Waste requiring special treatment
Sludge waste: 0.1-0.2 kg
(Residue after removal of impurities)
Waste liquid: 0.5 to 1.0 kg
(generated during the regeneration process of electrolyte)
Description.
Copper anode mud is a byproduct of the copper electrolytic refining process and mainly contains rare metals such as gold, silver, selenium, and tellurium. Copper concentrates are used as raw materials, and anode mud is generated during the purification process of copper in electrolytic refining. Although electricity consumption is relatively low, the process requires natural gas, and control of the electrolyte, which uses sulfuric acid, is critical.
tellurium (Te)
Input power
0.3~0.5 kWh/kg
(consumed in electrolysis and refining processes)
Input fuel
Natural gas: 0.1-0.2 kg
(used for heating process in refining furnaces)
CO₂ emissions other than combustion
0.05-0.1 kg CO₂/kg
(emissions from treatment of impurities and chemical reactions in the refining process)
Amount of change to resources such as soil and water
Copper anode mud: 10-30 kg
(Tellurium is recovered from copper electrolytic refining by-products)
Water: 3 to 5 L
(used for cooling and cleaning processes)
Input Item Name and Input Quantity Guideline
Copper anode mud: 10 to 30 kg
(tellurium concentration range of 0.1 to 1.0%)
Sulfuric acid: 0.5 to 1.0 kg
(Electrolyte composition)
Solvents and other process substances
Sulfuric acid solution: 5 to 7 L (circulation rate of at least 90%)
(for electrolysis and impurity removal)
Waste requiring special treatment
Sludge waste: 0.1 to 0.2 kg
(Residue after removal of impurities)
Waste liquid: 0.5 to 1.0 kg
(Treated sulfuric acid electrolyte)
Description.
Tellurium is primarily recovered as a byproduct of the copper electrolytic refining process. The production process typically involves extraction from copper anode mud and uses electricity or natural gas for refining and separation. Tellurium refining requires sulfuric acid electrolyte, which can be reused after use but requires some waste disposal.
Industrial phosphorus (Elemental Phosphorus)
Input power
0.8 to 1.2 kWh/kg
(used in electrolysis or reactor operation)
Input fuel
Coke (from coal): 1.5 to 2.0 kg
(as reducing agent)
Natural gas: 0.1 to 0.3 kg
(used for heating process)
CO₂ emissions other than combustion
0.6 to 1.0 kg CO₂/kg
(reduction reaction and by-products from the process)
Amount of change to resources such as soil and water
Phosphorus ore: 5-7 kg
(P₂O₅ concentration in concentrate: 25-35%)
Water: 2 to 4 L
(used for cooling and cleaning processes)
Input Item Name and Input Quantity Guideline
Phosphorus ore (concentrate): 5-7 kg
Silica sand (SiO₂): 1.0-1.5 kg
(used for slag formation)
Limestone (CaCO₃): 0.5-1.0 kg
(for removal of impurities)
Solvents and other process substances
Sulfuric acid: 0.2-0.5 kg (circulation rate >90%)
(involved in cleaning and phosphoric acid production)
Waste requiring special treatment
Slag (including silica): 1.0 – 2.0 kg
(Slag can be partially reused)
Waste solution: 0.5 to 1.0 kg
(Sulfuric acid solution must be treated)
Manufacturing Process Overview
Industrial phosphorus is produced primarily by the electric furnace process. In this process, phosphorus ore, coke, and silica sand are mixed and reduced in an electric furnace to produce phosphorus vapor, which is condensed to obtain phosphorus. The phosphorus content in the phosphate concentrate is important, and impurities are removed as slag along with silica and limestone.
Industrial arsenic
Input power
0.5 to 1.5 kWh/kg
(used for furnace operation and refining process operation)
Input fuel
Coke: 1.0 to 2.0 kg
(used as reducing agent)
Natural gas: 0.1 to 0.3 kg
(Used to heat process furnaces)
CO₂ emissions other than combustion
0.4~0.8 kg CO₂/kg
(generated from reduction reaction and impurity removal process)
Amount of change to resources such as soil and water
Arsenic ore: 3-5 kg
(As content in concentrate: approx. 20-30%)
Water: 2 to 3 L
(used for cleaning and cooling processes)
Input Item Name and Input Quantity Guideline
Arsenic concentrate: 3-5 kg
Iron oxide (Fe₂O₃): 0.5 to 1.0 kg
(to accelerate the reaction and produce slag)
Limestone (CaCO₃): 0.5-1.0 kg
(for removal of impurities)
Solvents and other process substances
Acid solution (H₂SO₄): 0.2-0.5 kg (circulation rate 80-90%)
(used for cleaning and refining)
Waste requiring special treatment
Slag (oxide mixture): 1.0 – 2.0 kg
(part of which can be recycled)
Waste liquid (acidic solution): 0.5 to 1.0 kg
(Neutralization treatment is required)
Manufacturing Process Overview
Arsenic is mainly produced from arsenic concentrates (e.g., arsenicals) using the reduction furnace method. Arsenic oxide (As₂O₃) is converted to metallic arsenic by heating with a reducing agent such as coke. Condensation of the arsenic vapor produced yields arsenic of high purity. Acidic solutions are often used during purification, and some of the slag produced is recycled.
Antimony (Sb)
Input power
1.5 to 2.5 kWh/kg
(used in furnace operation and refining process)
Input fuel
Coke: 1.2 to 2.0 kg
(used as reducing agent)
Heavy oil/natural gas: 0.2-0.4 kg
(used for furnace heating)
CO₂ emissions other than combustion
0.6~1.2 kg CO₂/kg
(generated from reduction process and removal of impurities)
Amount of change to resources such as soil and water
Antimony concentrate: 3-4 kg
(assuming 20-30% Sb content in concentrate)
Cooling water: 4-6 L
Input Item Name and Input Quantity Guideline
Antimony concentrate: 3-4 kg
Iron oxide (Fe₂O₃): 0.5-1.0 kg
(used for slag generation)
Limestone (CaCO₃): 0.8-1.2 kg
(for removal of impurities)
Solvents and other process substances
Sulfuric acid (H₂SO₄): 0.3-0.5 kg (circulation rate 90%)
(used for cleaning and impurity removal)
Waste requiring special treatment
Slag (oxide): 1.5 to 2.0 kg
(may contain reusable components)
Waste acid: 0.4-0.8 kg
(Neutralization treatment required)
Manufacturing Process Overview
Antimony is mainly produced using the reduction furnace method. Antimony concentrate (antimony sulfide ore, Stibnite) is heated with coke to produce antimony metal. In the refining process, impurities are removed using acid. The slag and waste acid produced are properly treated or recycled.
Arsenic Concentrate
Input power
1.0 to 2.0 kWh/kg
(used in smelting and concentrate treatment processes)
Input fuel
Coke: 0.5 to 1.0 kg
(Used as heat source and reducing agent in reduction furnaces)
Natural gas or heavy oil: 0.3 to 0.6 kg
(used for furnace heating and refining processes)
CO₂ emissions other than combustion
0.5~0.8 kg CO₂/kg
(generated from side reactions in the concentrate treatment process)
Amount of change to resources such as soil and water
Crude ore (ore): 5-6 kg
(assuming arsenic content of 5-10%)
Cooling water: 10-15 L
Input Item Name and Input Quantity
Arsenic-containing ore: 5-6 kg
(Typical examples: arsenopyrite, AsFeS, arsenic copper ore, etc.)
Iron oxide (Fe₂O₃): 1.0-2.0 kg
(used for slag generation)
Solvents and other process substances
Sulfuric acid: 0.3-0.5 kg (circulation rate 90%)
(used for cleaning and impurity removal)
Waste requiring special treatment
Slag (oxides): 2-3 kg
(Possibly reused due to metal oxide content)
Waste acid: 0.4-0.6 kg
(Neutralization treatment is required)
Manufacturing Process Overview
In the production of arsenic concentrates, arsenic-containing ores (such as arsenopyrite) are processed in a reduction furnace to obtain concentrates. In the reduction process, coke serves as the heat source and reducing agent. Natural gas or heavy oil may also be used to heat the furnace. The oxide slag and waste acid produced are properly treated and some can be recycled.
Iron oxide (Fe₂O₃)
Input power
0.5 to 1.2 kWh/kg
(used to operate firing furnaces and separation equipment)
Input fuel
Natural gas: 0.1 to 0.3 kg
(Used as fuel for heating)
Coke: 0.1 to 0.2 kg
(may be used in part as a reducing agent for oxygen removal)
CO₂ emissions other than combustion
0.2 to 0.5 kg CO₂/kg
(side reaction from ore processing and impurity removal)
Amount of change to resources such as soil and water
Iron ore: 1.5 to 2.0 kg
(Use concentrates with high iron content)
Cooling water: 5-10 L
Input Item Name and Input Quantity Guideline
Iron ore (concentrate)
Coal Coke
Input power
0.05~0.15 kWh/kg
(Used for measurement equipment, operation of conveying equipment, etc.)
Input fuel
Coal: 1.3 to 1.5 kg
(Used in dry distillation furnaces to remove volatile components)
Natural gas: 0.05-0.1 kg
(may be used for auxiliary heating of furnaces)
CO₂ emissions other than combustion
0.8 to 1.2 kg CO₂/kg
(gas emission during coal dry distillation process)
Amount of change to resources such as soil and water
Water (for cooling): 3-5 L
Input Item Name and Input Quantity
Coal (coking coal): 1.3 to 1.5 kg
(Coal with low volatile components is mainly used)
Solvents and other process substances
None
(No solvent is used in the dry distillation process)
Waste requiring special treatment
Tar: 0.05-0.1 kg
(Generated as a by-product, often used in the chemical industry)
Coke oven gas: approx. 0.3-0.5 m³.
(typically reused as fuel)
supplement
Coal coke is processed in a dry distillation oven (coke oven) at a high temperature of around 1,000℃. During this process, volatile components are released as gas, and the remaining solid material is coke.
By-products include tar and coke oven gas, which are reused as fuels and raw materials for chemicals.
Antimony 1kg
Input power
2.0~3.5 kWh/kg
(used in electrolytic refining and heat treatment processes)
Input fuel
Coal/coke: 0.8 to 1.2 kg
(Heating fuel in reduction furnace)
Natural gas: 0.1-0.2 kg
(may be used to preheat furnaces)
CO₂ emissions other than combustion
0.5~0.7 kg CO₂/kg
(generated from chemical reactions and decomposition of impurities in raw materials)
Amount of change to resources such as soil and water
Waste ore and slag: 5-8 kg
(Impurities generated in the ore processing process)
Water (for cooling and cleaning): 3-6 L
Input Item Name and Input Quantity Guideline
Antimony concentrate: 2.5 to 3.0 kg
(20-40% antimony content in ore)
Flux (lime, etc.): 0.3-0.5 kg
(promotes slag formation during melting)
Solvents and other process substances
Acid (sulfuric acid, hydrochloric acid, etc.): 0.2-0.4 L
(Impurity removal and refining process)
Circulation rate: 80-90%.
Waste requiring special treatment
Slag (furnace slag): 2-4 kg
(Special treatment is required due to the presence of toxic components)
Sulfur compounds in exhaust gas: 0.1 to 0.3 kg
(Exhaust gas treatment is required)
supplement
Antimony is generally produced by a reduction method using an oxidation-reduction furnace. When antimony metal is obtained from concentrates, antimony oxide is reduced with coke.
In the case of sulfide minerals, a roasting process is required before refining, where sulfur dioxide and other harmful gases are generated.
antimony concentrate
Input power
0.2 to 0.5 kWh/kg
(used in crushing and beneficiation processes)
Input fuel
Diesel oil: 0.05-0.1 kg
(as fuel for heavy equipment for mining and transporting ore)
Heat treatment with electricity and coal: total fuel energy equivalent to 0.1-0.2 kg
CO₂ emissions other than combustion
0.1 to 0.2 kg CO₂/kg
(Process emissions from beneficiation and washing treatment)
Amount of change to resources such as soil and water
Waste rock and tailings: 4-8 kg
(unused ore generated in the treatment process)
Water consumption: 5 to 10 L
(used for ore dressing and washing process)
Input Item Name and Input Quantity Guideline
Antimony-bearing ore: 5-8 kg
(Antimony content in the raw ore ranges from 10 to 20%)
Solvents and other process substances
Acid (hydrochloric or sulfuric acid): 0.1 to 0.3 L
(used in the flotation process)
Circulation rate: 80-90%.
Waste requiring special treatment
Tailings (waste ore): 3-5 kg
(Environmental treatment required)
Acidic wastewater: 0.1-0.3 L
(Wastewater treatment required)
supplement
For antimony concentrates, the flotation method is generally used, and the concentrates are beneficiated from the ore and sent to the next refining process.
After crushing, the ore mined in the mine is flotation screened to remove impurities to be converted to concentrate. This results in the majority of the mined coarse ore being discarded as tailings.
Bismuth 1kg
Power input (kWh/kg)
0.5 to 1.0 kWh/kg
(used in electrolytic refining and furnace refining processes)
Fuel input Type/amount
Coal: 0.2 to 0.4 kg
(Used as heat source in the reduction process)
Natural gas: 0.1 to 0.3 kg
(used for furnace heating)
CO₂ emissions other than combustion
0.05~0.1 kg CO₂/kg
(Process emissions in the refining process)
Amount of change to resources such as soil and water
Tailings (unused portion): 3-6 kg
(Residual impurities from crude ore)
Water consumption: 4 to 8 L
(used for cooling and cleaning)
Input Item Name and Input Quantity Guideline
Bismuth concentrate: 3-4 kg
(Amount depending on concentrate content)
Solvents and other process substances
Sulfuric acid: 0.05-0.1 L
(used for wet refining)
Circulation rate: 85-90%.
Catalyst use and degradation rate
not used
Waste requiring special treatment Name and quantity standard
Tailings waste: 2-4 kg
(generated as a byproduct of mining)
Acidic wastewater: 0.1-0.3 L
(Wastewater treatment required)
supplement
Bismuth is often produced as a smelting byproduct of lead and copper, using redox processes or hydrometallurgical methods. Inputs and energy usage may vary slightly depending on the raw materials used and the efficiency of the process.
Bismuth concentrate
Power input (kWh/kg)
0.4 to 0.8 kWh/kg
(used in ore crushing and flotation processes)
Fuel input Type/amount
Light and heavy oil: 0.1 to 0.3 kg
(Mining equipment operation and transportation)
Fuel consumption may fluctuate in many areas that rely on electricity-derived energy.
CO₂ emissions other than combustion
0.1-0.15 kg CO₂/kg
(indirect emissions from flotation chemical use and process wastewater)
Amount of change to resources such as soil and water
Tailings discharge: 5-10 kg
(waste of impurities in ore)
Water consumption: 6 to 12 L
(water required for flotation process and cleaning)
Input Item Name and Input Quantity Guideline
Bismuth-containing ore (before processing into bismuth concentrate): 3-6 kg
(varies depending on the concentration of bismuth in the ore)
Solvents and other process substances Name, amount used, and circulation rate
Flotation agents such as xylene and alkylbenzene: 0.05-0.1 kg
Circulation rate: about 90
(Some is reused, but needs to be replenished periodically)
Catalyst use and degradation rate
No catalyst is used.
Waste requiring special treatment Name and quantity standard
Tailings: 4-8 kg
(discarded as impurities in ore)
Flotation wastewater: 0.2 to 0.5 L
(Must be discharged after treatment)
supplement
In the production of bismuth concentrates, there are many ways to recover bismuth as a byproduct in concentrates such as copper, lead, and tin, which optimizes energy use in flotation and refining. The input energy figures also vary depending on the location of the mine and the level of modernization of the processing plant.
Industrial Carbon
Power input (kWh/kg)
0.5 to 2.0 kWh/kg
(used in graphitization process and carbon black production)
Fuel input Type/amount
Natural gas: 0.1 to 0.3 kg
(when used in high temperature reactions)
Heavy oil: 0.05-0.1 kg
(Used in some processes)
CO₂ emissions other than combustion
0.4~0.8 kg CO₂/kg
(byproduct of carbonization of raw materials and decomposition of volatile components)
Amount of change to resources such as soil and water
Wastewater volume: 1 to 2 L
(when used in cooling process)
Input Item Name and Input Quantity Guideline
Petroleum coke: 2 to 3 kg
(Used in the production of graphite and carbon fiber)
Pitch: 0.2 to 0.4 kg
(when used as a binder)
Solvents and other process substances Name, amount used, and circulation rate
Benzene solvents: 0.01 to 0.02 kg
Circulation rate: 90% or more
(Surface treatment and impurity removal in some processes)
Catalyst use and degradation rate
No catalysts are used (often using thermochemical processes)
Waste requiring special treatment Name/amount
Volatile organic compounds (VOCs): 0.1 to 0.2 kg
(treated as exhaust gas)
Drainage: 1 to 2 L
(Cooling water drainage treatment required)
supplement
Industrial carbon (e.g., carbon black, graphite) is produced from petroleum coke or pitch, primarily through thermochemical reactions. Because the production process requires energy-intensive high-temperature processing, control of fuel type and power input is important.
Industrial silicon
Power input (kWh/kg)
10-15 kWh/kg
(used for reduction of silicon metal in arc furnaces)
Fuel input Type/amount
Charcoal or coke: 0.6 to 1.0 kg
(Used to reduce silica)
CO₂ emissions other than combustion
1.5~2.0 kg CO₂/kg
(generated as a byproduct of the silicon reduction reaction)
Amount of change to resources such as soil and water
Water consumption: 5 to 10 L
(used for cooling process, etc.)
Input Item Name and Input Quantity Guideline
Silica (SiO₂) concentrate: 2.5 to 3.0 kg
(obtained from quartz)
Solvents and other process substances Name, amount used, and circulation rate
Cooling water: 5-10 L
Circulation rate: 85-90
(Used for cooling process)
Catalyst use and degradation rate
No catalyst (not typically used in the production of metallic silicon)
Waste requiring special treatment Name and quantity standard
Silicate slag: 1.0-1.2 kg
(generated as a byproduct of furnace operation)
Wastewater: 5 to 10 L
(Discharged as cooling water after treatment)
supplement
Industrial silicon (silicon metal) is obtained by reducing quartz (silica)-based raw materials in an arc furnace together with coke and charcoal. Since reduction in a high-temperature environment is necessary, a large amount of electric power is required. Silicate slag is generated as a byproduct, and wastewater and CO₂ management is also important in the manufacturing process.
Rare earth concentrates
Approximate composition of typical rare-earth concentrates (per tonne)
Light Rare Earth Elements (LREE)
Cerium (Ce): 45-50
Lantern (La): 20-25
Neodymium (Nd): 15-20
Praseodymium (Pr): 5-7
Samarium (Sm): 1-3
Heavy Rare Earth Elements (HREE)
Gadolinium (Gd): 1-2%.
Dysprosium (Dy): 0.5 to 1.5
Erbium (Er): 0.3 to 0.8
Holmium (Ho): 0.05-0.1%
Thulium (Tm): 0.01-0.05
Ytterbium (Yb): 0.3-0.6
Lutetium (Lu): 0.01-0.05
Other
Yttrium (Y): 3-8% (often classified as heavy rare earths)
Characteristics by Ore
Bastnesite: Cerium predominates, with neodymium and praseodymium also relatively common.
Monazite: high in cerium and lanthanum, but low in heavy rare earths.
Ion adsorption ore (southern China): high percentage of heavy rare earths (especially dysprosium and yttrium).
summary
Of a ton of rare earth concentrates, 45-50% is composed of cerium, followed by neodymium and lanthanum. Heavy rare earths account for less than 10% of the total, but are in high demand, especially dysprosium and yttrium, as they play an important role in the high-tech sector.
These percentages may vary depending on the actual mining location and processing process, so specific deposit data is needed for an accurate analysis.
The metallic content of rare earth elements in a ton of rare earth ore varies depending on the type of ore mined and its quality, but the REO (Rare Earth Oxides) content in a typical concentrate is said to range from 5 to 15% by weight. This can be converted to metal weight as follows
Calculation of rare earth metal components (e.g., 10% by weight)
Percentage of rare earth oxides: 10% (100 kg of REO in 1 ton)
Oxide to metal conversion factor: approx. 0.85 (metal weight with oxygen removed from oxide)
Calculation Example:
REO 100 kg × 0.85 ≈ 85 kg
→ 85 kg of rare earth metals per ton of ore
Approximate content of each type of ore
Bastnesite: High-grade ore with REO content of approximately 60-70%.
Monaz stone: 40-60% REO content.
Ion adsorption ore (South China): REO content is as low as 0.1% to 1%, but heavy rare earths are abundant.
summary
A typical ton of rare-earth concentrate contains approximately 50-150 kg of rare-earth oxides (REO).
In terms of metal, the calculation yields about 42.5-127.5 kg of rare earth metals.
Actual production volumes will vary depending on ore grade, processing process, and separation efficiency.
To produce 1 ton of rare earth concentrate (typically containing 60-70% rare earth oxides, REOs), approximately 5-8 tons of raw ore need to be mined, depending on the ore grade. Key mining operations include crushing, grinding, flotation, and acid leaching, with Bayan Obo and Mountain Pass providing benchmarks for process estimates.
The energy requirement ranges between 200-400 kWh per ton of concentrate, with heavy use of electricity in grinding and solvent extraction steps. Acid roasting and leaching consume significant chemicals like sulfuric acid and hydrochloric acid. Acid roasting and leaching consume significant chemicals like sulfuric acid and hydrochloric acid.
Waste generation includes tailings from beneficiation and radioactive waste from monazite-containing ores. Typically, 20-40 tons of tailings are produced per ton of concentrate. Typically, 20-40 tons of tailings are produced per ton of concentrate
The amount of radioactive material contained per ton of rare earth concentrate depends on the type of ore. Ores such as monazite and bastnaesite contain thorium (Th) and uranium (U). In general, several tens of kilograms of radioactive material may be produced per ton of concentrate, specifically 20 to 30 kg of thorium and 5 to 10 kg of uranium.
This radioactive material must be managed during the treatment process and must be disposed of or reused appropriately.
Radioactive Material Processing
Power input: 2-5 kWh/kg
Fuel input: 0.1-0.2 kg natural gas, 0.05-0.1 kg heavy oil
CO2 emissions other than combustion: 0.01-0.05 kg
Resource change in soil and water: 0.2-0.5 kg
Input: 0.2 kg lime, 0.1-0.15 kg acid (e.g., sulfuric acid)
Solvent: Nitric acid-based 0.05-0.1 kg, circulation 85%.
Waste: 0.05 kg radioactive liquid waste, 0.02-0.03 kg solid waste
When zeolites or similar materials are used as catalysts, the degradation rate is 5-10%.
Radioactive materials in the world’s rare-earth ores are mainly thorium and uranium. The amount of radioactive materials per ton of rare-earth concentrate is estimated to be approximately 500 to 1500 grams. However, it varies greatly depending on the type of deposit and the producing country, with thorium often predominating.
It should also be noted as a radioactive waste that requires treatment and storage, and strict controls are required to ensure that these materials do not affect the environment during refining and wastewater management.
Typical separation procedure for rare earth elements with solvent and pH adjustment
Below is a general procedure for separating typical REEs (Rare Earth Elements) into Light REEs (LREE), Heavy REEs (HREE), and Medium REEs by solvent extraction. Details of solvents and pH adjusters used in each step are also included.
- concentrate pretreatment
Purpose: Leaching of rare earth elements from rare earth ores (monazite, bastnaesite, etc.).
Method: Rare earths are converted to solution by acid leaching (sulfuric acid and hydrochloric acid).
Waste: Residues (may contain radioactive materials).
- separation of light rare earths (LREE: La, Ce, Pr, Nd, etc.)
Solvents used:
HDEHP (diethylhexyl phosphate)
TBP (tributyl phosphate)
pH adjuster:
Sodium hydroxide (NaOH), sodium carbonate (Na₂CO₃)
Approximate amount used: about 30-50 kg per ton of concentrate
Procedure:
**Selective extraction of La and Ce using solvents in an acidic environment (pH 2-3)**.
Pr and Nd are separated at the next stage of pH (3.5-4).
- separation of medium rare earths (Gd, Tb, Dy)
Solvents used:
P507 (2-ethylhexyl phosphate mono-2-ethylhexyl ester)
HDEHP combination
pH adjuster:
Ammonia water (NH₄OH) or sodium hydroxide (NaOH)
**Use
Separation procedures for all rare earth elements (light, medium, and heavy rare earths)
Below is a process for solvent extraction that efficiently separates rare-earth elements, along with typical procedures and the amount of pH adjusters used. In particular, procedures incorporating the separation of medium rare earths (Gd, Tb, Dy, etc.) are included.
- concentrate pretreatment
Objective: To leach rare earth elements from monazite and bastnaesite.
Method: Acid leaching using sulfuric acid or hydrochloric acid to turn rare earths into solution.
Waste: Residues, possibly including radioactive materials.
- separation of light rare earths (LREE)
Representative elements: La (lanthanum), Ce (cerium), Pr (praseodymium), Nd (neodymium)
Solvents used
HDEHP (diethylhexyl phosphate)
TBP (tributyl phosphate)
pH adjuster
Sodium hydroxide (NaOH)
Sodium carbonate (Na₂CO₃)
Approximate amount used: 30-50 kg per ton of concentrate
Procedure: 1.
pH 2-3: La and Ce are preferentially extracted.
pH 3.5-4: Pr and Nd are separated in the next step.
- separation of medium rare earths (MREE)
Representative elements: Gd (gadolinium), Tb (terbium), Dy (dysprosium)
Solvents used
P507 (phosphoric acid monoester solvent)
HDEHP (applicable to residual liquid from light rare earth separation)
pH adjuster
Ammonia water (NH₄OH)
Approximate amount used: 10-20 kg per ton of concentrate
Procedure: 1.
Separation of Gd and Tb from other elements under pH 4-5 conditions.
Gradually increase pH to separate Dy.
- heavy rare earth (HREE) separation
Representative elements: Ho (holmium), Er (erbium), Yb (ytterbium), Lu (lutetium)
Solvents used
D2EHPA (phosphate solvent)
Accuracy is improved when used with P507.
pH adjuster
Sodium hydroxide (NaOH)
Quantity used: 20-30 kg per ton of concentrate
Procedure: 1.
Stepwise separation of each element at pH 5-6.
The final stage of HREE uses solvent exchange and re-extraction.
- pH adjuster used and circulation rate
Main pH adjusters: NaOH, NH₄OH
Recirculation rate: Some of the conditioner can be circulated, but about **50-70%** of the amount used will be consumed.
Total amount used: A total of 50-100 kg of pH adjuster is required per ton of concentrate.
- waste per ton of concentrate
Acid residues: May contain radioactive waste. Can reach several hundred kilograms per ton of concentrate.
Solvent compensation: **2-5%** of solvent is consumed per ton of concentrate processed (in units of several kilograms).
This process allows stepwise separation of rare earth elements from light rare earths to heavy rare earths. The separation of medium rare earths requires the combination of specific solvents and precise pH adjustment, which must be optimized at each step.
Incorporation of Eu, Tm, and Y in the Separation of Rare Earth Elements and the Removal of Radioactive Materials
- separation and incorporation procedure for Eu, Tm, and Y
The separation of rare earth elements is divided into light rare earths (LREE), medium rare earths (MREE), and **heavy rare earths (HREE)** as follows: Eu (europium), Tm (thulium), and Y (yttrium) are classified as medium and heavy REE and are separated in different pH ranges.
Eu (europium) separation
Classification: Near light rare earths but with unique properties. Separated from other LREEs by their redox properties.
Separation method:.
Solvent extraction (e.g. HDEHP) in the pH range 3.5-4
Reduction of Eu³⁺ to Eu²⁺ by redox reaction and separation
Tm (thulium) separation
Classification: Heavy Rare Earth (HREE)
Separation procedure: use of D2EHPA or P507.
Tm is also separated at the same stage as other HREEs (Ho, Er, Lu) which are separated at pH 5-6.
Y (yttrium) separation
Classification: Heavy Rare Earth (HREE)
Solvents: P507, D2EHPA.
Procedure: 1.
Y is separated with HREE, with particular emphasis on separation because of its use in applications close to Nd and Dy.
- removal and disposal of radioactive materials
Radioactive materials (mainly uranium and thorium) are usually removed prior to solvent extraction by
Sedimentation process after acid leaching: 1.
Sulfuric acid and carbonic acid are used to precipitate uranium and thorium.
The precipitated radioactive materials are disposed of separately and safely.
Ion exchange method: Ion exchange method
Some refineries use ion-exchange resins to remove uranium and thorium from rare-earth solutions.
Radioactive waste generated:.
It is common for each ton of rare earth concentrate to generate several kilograms of radioactive waste. This is an important environmental management issue.
Summary: Positioning of Eu, Tm, and Y in the procedure
Eu: Classified as light rare earths, but specifically separated utilizing redox.
Tm, Y: extracted over a similar pH range as part of the heavy rare earths.
Removal of radioactive materials: Radioactive materials are removed by precipitation or ion-exchange methods in the preliminary stage of solvent extraction, which makes the later stage of solvent extraction more efficient.
Through this process, all rare-earth elements are separated step by step and utilized for precision applications.
Silica concentrate
Composition of inputs, energy, and waste
Input power
0.4-1.5 kWh/kg
(used in crushing, beneficiation, washing, and drying processes)
Input fuel
Light oil or natural gas: 0.1-0.3 kg/kg
(for drying process or as heat source)
CO2 other than combustion
Approx. 0.01-0.05 kg CO₂/kg
(possible emissions from chemical decomposition during cleaning)
Changes made to resources such as soil and water
Ore residue (impurities): 1 to 2 kg/kg
(residue after beneficiation from ore)
Water consumption: 3-5 L/kg (washing process)
Input materials and their approximate amounts
input
Silica ore (e.g., quartz ore): 1.5-3 kg/kg
(remove impurities from mined ore)
Solvents and other process substances
Acid (e.g., hydrofluoric acid): 0.01-0.05 kg/kg
Circulation rate: 80-90%
(used to remove trace amounts of iron, etc.)
Waste requiring special treatment
Ore residue: 1 to 2 kg/kg
(impurities separated in ore dressing. Mainly lime, iron, etc.)
Spent solvent/acid neutralization waste: 0.05-0.1 kg/kg
Process Overview
Mining: quartz and silica ores.
Crushing and washing: The ore is crushed and impurities are washed out with acid or water.
Beneficiation: Separation of target silica by specific gravity or chemical treatment.
Drying: Water is removed and the product is shipped as concentrate.
High purity of the ore is important in the production of silica concentrates, and energy is required for acid treatment, crushing, and drying. The treatment of ore residues is also required as part of environmental management.
germanium (Ge)
- energy input
Power input
approx. 50-70 kWh/kg (consumed in refining, electrolytic refining and distillation processes)
Input fuel
Natural gas: 0.1-0.3 kg/kg (heating applications in refining processes)
- CO₂ emissions other than combustion
Approx. 0.1-0.3 kg CO₂/kg (emissions from solvent reaction during purification)
- impacts on resources such as earth, rocks, and water
Ore residue (impurities): 15-25 kg/kg (large amount of residue due to germanium concentrate content of less than 1%)
Water consumption: 10-20 L/kg (for leaching and washing)
Ingredients and usage
Raw ore: zinc ore concentrate, by-products from coal ash
Ore: 50-100 kg/kg (depending on germanium content of concentrate)
Solvents and process substances
Sulfuric acid: 1.0-2.0 kg/kg (used for leaching treatment)
Hydrochloric acid: 0.5-1.0 kg/kg (removal of impurities)
Circulation rate: 90% or more
- catalyst and degradation rate
Reduction catalyst: copper sulfate, etc.
Amount used: 0.1-0.5 kg/kg
Degradation rate: 5-10%/kg
- waste requiring treatment
Ore residue/ash: 15-25 kg/kg
Acid-treated effluent: 5-10 kg/kg (requires neutralization)
Manufacturing Process Overview
Ore leaching: Leaching germanium from zinc concentrates and coal ash using sulfuric acid and hydrochloric acid.
Liquid-liquid extraction: Remove impurities by solvent extraction to obtain a germanium-containing solution.
Reduction refining: Reduction of germanium oxide to germanium metal using a reduction catalyst.
Distillation or Electrolysis: Distillation or electrolytic purification is used to obtain high-purity germanium.
This production process is characterized by a high generation of ore residues due to the low content of raw materials. In addition, process substances such as sulfuric acid and hydrochloric acid are often used, and the recycling and effluent treatment of these substances is important.
Tin
- energy input
Power input:
1-3 kWh/kg (used for concentrate reduction process and electrolytic refining)
Input fuel:
Coal or coke: 0.5 to 1.0 kg/kg (heat source during reduction from concentrate)
- CO₂ emissions other than combustion
0.1-0.3 kg CO₂/kg (due to chemical reactions in the reduction process)
- impacts on resources such as earth, rocks, and water
Ore residue: 5-20 kg/kg (waste impurities in concentrate)
Water consumption: 15-30 L/kg (for ore leaching and washing)
- input materials and estimated usage
Raw material ore: Tinite (SnO₂) concentrate
Ore quantity: 10-20 kg/kg (for tin content of 20-30% in concentrate)
- solvents and process substances
Acid (sulfuric or hydrochloric acid): 0.5-1.0 kg/kg (for removal of impurities)
Circulation rate: 80-90% (some acid is circulated and reused)
- catalyst use and degradation rate
Catalyst:
When used for reduction, copper sulfate or activated carbon may be used.
Amount used: 0.1-0.3 kg/kg
Degradation rate: 5-10%/kg
- waste requiring treatment
Tailings and slag: 10-15 kg/kg (ore-derived impurities)
Acidic liquid waste: 5-10 kg/kg (requires appropriate neutralization treatment)
Manufacturing Process Overview
Ore refining:
impurities are removed from tinstone (SnO₂) to obtain a concentrated concentrate.
Reduction process:
concentrate is reduced at high temperature with coke or coal to produce metallic tin.
Refining and electrolysis:
Electrolytic refining may be used to obtain high-purity tin.
The process generates a large amount of tailings due to the low tin content of the raw ore. The reduction process consumes a lot of energy and requires efficient use of electricity and fuel. In addition, the recycling of the acid used and waste disposal are environmental issues.
Tinstone (SnO₂) concentrate
- energy input
Power input:
0.3-1.5 kWh/kg (used in ore dressing machines and ore crushing processes)
Input fuel:
Light or heavy oil: 0.1-0.3 kg/kg (fuel for excavators and trucks)
Fuel (e.g., coal) for electricity substitution: 0.05-0.1 kg/kg (drying process)
- CO₂ emissions other than combustion
0.05-0.15 kg CO₂/kg (emissions from process-derived carbonate decomposition and machine operation)
- changes in resources such as earth, rocks, and water
Waste rock or slag: 3-10 kg/kg (valueless material in raw ore)
Water consumption: 50-100 L/kg (for ore washing and flotation beneficiation)
- input materials and estimated usage
Raw material ore: Tin ore (tin content of about 0.5 to 2%)
Amount of ore: 50-200 kg/kg (to obtain 1 kg of concentrate)
- solvents and process substances
Flotation agent (e.g., xylene): 0.01-0.05 kg/kg
Circulation rate: 70-80%.
- catalyst use and degradation rate
Not applicable as catalysts are not normally used in tin ore beneficiation.
- waste requiring treatment
Tailings: 3-10 kg/kg (waste rock from flotation process)
Contaminated water: 10-20 L/kg (effluent after chemical treatment)
Manufacturing Process Overview
Ore mining:
mining tin ore from the mine and preparing it for concentrates.
Beneficiation (flotation beneficiation method):
The ore is crushed and the tinstones (SnO₂) are sorted using flotation technology.
Concentration and washing:
The sorted tinstones are concentrated and washed, and finally shipped as concentrates.
This process generates large amounts of waste rock and contaminated water due to the low tin content in the raw ore. Efficient recycling of the flotation agent is also an important issue.
Lead (Lead, Pb)
Production process for 1 kg of lead (e.g., calcination/reduction method)
Lead is mainly produced from **sulfide ore (galena: PbS)** and lead concentrates are converted to lead oxide (PbO) by calcination and then refined to metallic lead in a reduction furnace.
Inputs and energy guidelines
Input power
Electricity: 0.2-0.3 kWh/kg
(auxiliary process for reduction and refining facilities)
Input fuel
Coke or coal: 0.4-0.6 kg/kg
(reduced reaction fuel)
CO₂ emissions other than combustion
CO₂ emissions: 0.3-0.5 kg/kg
(from carbonate components in ore and reduction process)
Amount of change in resources such as soil and water
Waste rock and slag: 4-5 kg/kg
(residual impurities from galena ore)
Raw material input
Lead concentrate (PbS content 80-85%): 1.5-2 kg
(required to produce 1 kg of lead)
Amount of solvents and process substances used
Flux (limestone and silicate): 0.1-0.2 kg/kg
(for slag formation)
Circulation rate: 60-80%
(some solvents and other materials are reused)
Amount of waste disposed
Waste slag: 0.8-1.2 kg/kg
(generated in the reduction process)
Dust and exhaust gas treatment waste: 0.05-0.1 kg/kg
Catalysts
Catalysts are generally not used in lead refining, but desulfurization catalysts and other catalysts may be used in the gas treatment process for environmental purposes.
summary
The production of 1 kg of lead requires 1.5-2 kg of lead concentrate, 0.5 kg of coke, and about 0.3 kWh of electricity, which inevitably results in CO₂ emissions and slag formation. Efficient process management and waste disposal are critical to reducing the environmental impact of lead production.
lead concentrate
- input power
Electricity consumption: 0.3-0.5 kWh/kg
(used for crushing and beneficiation processes)
- input fuel
Fuel type and amount used:
Diesel fuel (for mining equipment): 0.05-0.08 kg/kg
Coal or gas derived from electric power (for treatment plants): 0.1-0.2 kg/kg
CO₂ emissions other than combustion
CO₂ emissions: 0.05-0.1 kg/kg
(generated during waste rock treatment and transportation processes)
- the amount of change in resources such as earth, rocks, and water
Waste rock and slag: 3-5 kg/kg
(removal of impurities prior to beneficiation)
Water consumption: 5-8 kg/kg
(used in beneficiation and washing processes)
- input
Raw ore (galena ore): 2 to 2.5 kg
(approx. 50-70% lead in ore)
- use of solvents and process substances
Limestone (for flotation): 0.05-0.1 kg
Chemicals for floss (e.g., xylene-based): 0.01-0.02 kg
Circulation rate: 60-70%
(beneficiation chemicals are partially reused)
- waste requiring special treatment
Waste rock: 1-3 kg/kg
(rock remaining after beneficiation)
Beneficiated wastewater (pollution treatment required): 2 to 3 kg/kg
explanation
The production of lead concentrate requires the mining of galena ore (PbS) and a beneficiation process. Impurities are separated using ore crushing and flotation methods to obtain concentrates with high lead content. The fuel and electricity input depends on the efficiency of the mining and beneficiation process, and the beneficiation chemicals are partially recirculated. Waste rock and beneficiation wastewater are the most important waste products.
Because of the environmental impact of this process, wastewater treatment and waste management measures are essential.
Boric acid (H₃BO₃)
- input power
Electricity consumption: 1.5 to 2.5 kWh/kg
(used in evaporation and concentration and crystallization processes)
- input fuel
Fuel type and amount used:
Natural gas: 0.3-0.5 kg/kg
(used for heating during heat treatment and evaporation)
CO₂ emissions other than combustion
CO₂ emissions: 0.1-0.15 kg/kg
(generated from carbonate reaction)
- amount of change in resources such as earth, rocks, and water
Water consumption: 3-5 kg/kg
(required for extraction and washing processes)
Amount of ore waste: 5-8 kg/kg
(separated as impurities)
- input
Typical raw materials:
Borac ore (B₂O₃ content approx. 30-40%): 3-4 kg
(used for conversion to boric acid)
- use of solvents and process substances
Sulfuric acid: 0.1-0.2 kg/kg
(acid treatment for boric acid extraction)
Circulation rate: 70-80%
(some acid is reused)
- waste requiring special treatment
Sludge (insoluble impurities): 1 to 2 kg/kg
(byproduct of Borac ore processing)
Acid-treated wastewater: 2-3 kg/kg
(requires proper neutralization and treatment)
explanation
Boric acid is produced from boron-containing ores, such as borac ore, by acid processing. In a typical process, the ore is crushed and the boron components are extracted using sulfuric acid, then evaporated and crystallized to yield boric acid. Large amounts of water and energy are required during the process, and some acids are circulated for use.
This manufacturing process generates sludge containing impurities and acidic wastewater, so proper waste treatment is important to reduce environmental impact.
Pollack concentrate (boron concentrate)
- input power
Electricity consumption: 0.5-1.0 kWh/kg
(used for ore crushing, ore dressing, and drying processes)
- input fuel
Fuel type and amount used:
Natural gas: 0.2-0.3 kg/kg
(used for drying in beneficiation process)
CO₂ emissions other than combustion
CO₂ emissions: 0.05-0.1 kg/kg
(generated by chemical processing of ore)
- the amount of change to resources such as earth, rocks, and water
Water consumption: 10-15 kg/kg
(used for ore dressing and impurity removal)
Amount of waste rock: 5-8 kg/kg
(removed during beneficiation process)
- input
Typical raw materials:
Boron ore (B₂O₃ content 20-30%): 4-6 kg
- solvents and process substances
Chemicals used: Sulfuric acid and sodium carbonate
Amount used: 0.1-0.2 kg/kg
Circulation rate: 60-80%.
- waste requiring special treatment
Impurity sludge: 2-3 kg/kg
(waste containing insoluble components)
Chemically treated wastewater: 5-10 kg/kg
(Needs neutralization and treatment)
explanation
Pollack concentrate is obtained from boron-bearing ores (such as bollack). The ore is crushed and impurities are removed by ore dressing. The boron components are then separated and concentrated using chemicals such as sulfuric acid and sodium carbonate. This process requires large amounts of water and generates sludge and chemical wastewater as byproducts.
Although some chemicals are circulated in the beneficiation and chemical treatment processes, proper waste management and treatment are essential. Effective beneficiation and concentration from the raw ore is required to obtain concentrates.
aluminum ingot
- input power
Electricity consumption: 13-15 kWh/kg
(electrolysis by Hall-Eroux method)
- input fuel
Fuel type and amount used:
Natural gas: 0.3-0.4 kg/kg
(dried and calcined in alumina production)
Heavy oil: 0.2-0.3 kg/kg
(used in some calcinations)
CO₂ emissions other than combustion
Emissions: 0.5-1.0 kg/kg
(emissions from calcium carbonate)
- amount of change in soil and water resources
Bauxite ore: 4-5 kg/kg
(50% alumina content)
Water consumption: 2-4 kg/kg
(cooling and refining process)
- input
Bauxite ore: 4-5 kg
Sodium carbonate or caustic soda: 0.1-0.2 kg
(used for alumina extraction)
- solvents and process substances
Name: Aluminum soda (AlF₃), Sodium soda (NaF)
Amount used (molten salt): 1 to 1.2 kg/kg
Circulation rate: 85-90%
(circulation of molten salt in the electrolysis cell)
- electrode usage and degradation rate
Name: Carbon electrode (anode)
Amount used: 0.4-0.5 kg/kg
Degradation rate: Wear and tear due to oxidation (20-25%)
- waste requiring special treatment
Name: Bauxite residue
Approximate quantity: 2 to 3 kg/kg
Manufacturing Process Overview
Alumina production: Bauxite ore is processed to extract alumina (Al₂O₃).
Electrorefining:
Alumina is dissolved in molten salt (sodium soda and aluminum soda) and decomposed into aluminum and oxygen through electric current. A carbon electrode reacts with the oxygen to produce CO
Aluminum recovery: Molten aluminum is extracted and turned into ingots.
Efficient energy use is essential in the aluminum production process because of its high power consumption. The depletion of carbon electrodes also contributes to CO₂ emissions, while the reuse of circulating salt contributes to the reduction of resource consumption.
Aluminum fluoride (AlF₃)
- input power
Electricity usage: 5-7 kWh/kg
(for process heating and control)
- input fuel
Fuel type and amount used:
Natural gas: 0.1-0.15 kg/kg
(used in drying process)
CO₂ emissions other than combustion
Emissions: 0.5-0.8 kg/kg
(from hydrofluoric acid and byproducts during reaction)
- amount of change in soil and water resources
Water consumption: 1 to 2 kg/kg
(used for washing and as solvent)
- input
Name: Alumina (Al₂O₃)
Feed rate: 0.5-0.6 kg/kg
Application: Aluminum supply source
Name: Hydrofluoric acid (HF)
Feed rate: 1.5 to 2.0 kg/kg
- solvents and process substances
Name: Water
Amount used: 1 to 2 kg/kg
Circulation rate: 80-90%.
- catalyst
Not used (no specific catalyst is required for this process)
- waste requiring special treatment
Name: Waste fluoride produced as a byproduct
Approximate quantity: 0.2-0.3 kg/kg
Manufacturing Process Overview
Reaction of raw materials:
Alumina (Al₂O₃) reacts with hydrofluoric acid (HF) to produce aluminum fluoride (AlF₃).
Filtration and drying:
After removing byproducts, the aluminum fluoride is dried.
Purification:
If necessary, additional washing or heat treatment is performed to remove impurities.
Aluminum fluoride is an important component in the electrorefining of aluminum and is used primarily in electrolyzers. Proper management of fluorinated gases and hydroxide waste is required during the production process.
Sodium fluoride (NaF)
- input power
Electricity consumption: 0.5-1.5 kWh/kg
(used for heating and stirring processes)
- input fuel
Fuel type and amount used:
Natural gas: 0.05-0.1 kg/kg
(used for heating in drying and refining processes)
CO₂ emissions other than combustion
Emissions: 0.1-0.3 kg/kg
(carbon dioxide produced during the process)
- the amount of change to resources such as earth, rocks, and water
Water consumption: 1 to 2 kg/kg
(water for washing and reaction)
- input
Name: Sodium hydroxide (NaOH)
Feed rate: 0.4-0.6 kg/kg
Applications: Sodium source
Name: Hydrofluoric acid (HF)
Input amount: 0.8 to 1.0 kg/kg
Applications: Fluorine source
- solvents and process substances
Name: Water
Amount used: 2 to 3 kg/kg
Circulation rate: 80-90%.
- catalyst
No catalyst used (no catalyst is used in normal sodium fluoride production)
- waste requiring special treatment
Name: Waste fluid containing fluoride
Approximate quantity: 0.1-0.3 kg/kg
Treatment method: Neutralization or decomposition at a dedicated treatment facility
Manufacturing Process Overview
Reaction:
Sodium hydroxide (NaOH) reacts with hydrofluoric acid (HF) to form NaF and water.
Filtration and washing:
The sodium fluoride produced is filtered and washed with water to remove impurities.
Drying:
Drying NaF crystals and processing them into powder or granular form.
Sodium fluoride is used in toothpaste and water fluoridation processes, as well as in industrial processes. In the manufacturing process, proper treatment of the byproduct, fluoride effluent, is important.
gallium (Ga)
- input power
Electricity consumption: 300-600 kWh/kg
(gallium electrorefining and extraction process)
- input fuel
Type and amount used:
Natural gas: 0.1-0.2 kg/kg
(used for heating process)
Heavy oil: 0.05-0.1 kg/kg
(part of pre-processing from concentrate)
CO₂ emissions other than combustion
emissions: 0.5 to 1.0 kg/kg
(secondary CO₂ emissions during the reaction)
- the amount of change to resources such as earth, rocks, and water
Water consumption: 10-15 kg/kg
(dissolution and washing processes)
Soil and stone residue: 2-3 kg/kg
(discarded in ore processing)
- input
Name: Bauxite concentrate (containing Al₂O₃)
Input: 10-50 kg/kg
Application: Extraction of gallium from byproducts of aluminum smelting
Name: Sodium hydroxide (NaOH)
Input amount: 1.0-1.5 kg/kg
Application: Dissolution and extraction of bauxite
Name: Sulfuric acid (H₂SO₄)
Input amount: 1.0 to 2.0 kg/kg
Applications: Purification of gallium
- solvents and process substances
Name: Organic solvent (acidic extractant)
Amount used: 0.05-0.1 kg/kg
Circulation rate: 90-95%.
- catalyst
No catalyst used (no catalyst required for normal gallium extraction)
- waste requiring special treatment
Name: Alkaline waste liquid
Approximate quantity: 1-3 kg/kg
Treatment method: Neutralizing treatment
Manufacturing Process Overview
Raw material acquisition:
Gallium is produced as a byproduct during the refining of aluminum from bauxite ore.
Solution Concentration and Extraction:
Gallium is concentrated using sodium hydroxide and extracted with organic solvents from the mother liquor remaining from the aluminum electrolysis process.
Purification:
The extracted gallium is electrolytically purified to obtain high-purity gallium.
The main organic solvents used for gallium extraction are
Ketone solvents
Typical example: MIBK (Methyl isobutyl ketone)
Applications: Used to selectively extract gallium from acidic solutions.
Features: High gallium extraction efficiency and solvent reuse.
D2EHPA (Di(2-ethylhexyl) phosphoric acid)
Applications: Used to separate gallium from other metals (zinc, aluminum, etc.).
Characteristics: A phosphate ester-based extractant that enables selective extraction of metal ions under acidic conditions.
TBP (Tributyl phosphate)
Use: Used to concentrate gallium from acidic solutions as part of solvent extraction.
Characteristics: A phosphate ester solvent widely used in metal recovery from acidic solutions.
Approximate Amount of Compensation
Amount of extractant supplement: About 1 to 3 kg of extractant is used per ton of gallium-containing solution.
Circulation rate: 90-95% is typical, but needs to be replenished for deterioration.
These solvents are used in multi-step extraction methods and are essential for efficient separation and concentration from gallium-containing solutions. Adjustment of pH and control of acidity within the process are important to improve separation efficiency.
The process relies primarily on the bauxite smelting process, which requires efficient recovery of gallium from the byproducts. Since alkali and sulfuric acid are used extensively during the refining process, the treatment of the effluent is important.
indium (In)
- input power
Electricity consumption: 150-300 kWh/kg
(refining process, electrolytic extraction)
- input fuel
Type and amount used:
Natural gas: 0.1-0.2 kg/kg
(used for drying and thermal treatment processes)
Heavy oil: 0.05-0.1 kg/kg
(used for heating during ore concentrating)
CO₂ emissions other than combustion
Emissions: 0.3-0.5 kg/kg
(byproducts from dissolution and refining processes)
- the amount of change to resources such as earth, rocks, and water
Water consumption: 5-10 kg/kg
(dissolution, washing)
Waste ore residue: 3-5 kg/kg
(residue after ore processing)
- input
Name: Zinc concentrate (indium content of approx. 0.1-0.2%)
Input: 500-1000 kg/kg
Application: Recovery of indium as a byproduct of zinc smelting
Name: Sulfuric acid (H₂SO₄)
Input: 2 to 3 kg/kg
Applications: Dissolution of zinc concentrates, separation of indium
Name: Hydrochloric acid (HCl)
Input amount: 1 to 2 kg/kg
Application: Removal of impurities from indium
- solvents and process substances
Name: Organic extractant
Amount used: 0.05-0.1 kg/kg
Circulation rate: 90-95%.
- catalyst
Not used (usually no catalyst is needed, but acid control is important for impurity removal)
- waste requiring special treatment
Name: Acidic liquid waste
Approximate quantity: 2-4 kg/kg
Treatment method: Neutralization and safety treatment
Manufacturing Process Overview
Zinc concentrate processing:
Electrolyzer sludge, a byproduct of zinc smelting, is used to recover the indium contained in zinc concentrates.
Acid treatment and dissolution:
Sludge is dissolved in sulfuric and hydrochloric acid to remove impurities and selectively extract indium.
Purification and Electrolysis:
After extraction, indium is electrolytically purified to obtain high-purity indium.
The process relies on a zinc smelting process, and indium is obtained as a byproduct of that process, so recovery efficiency and the purification process are critical. Because of the large quantities of acids and organic solvents used, liquid waste treatment is an integral part of the manufacturing process.
The following substances are commonly used as organic extractants in the production of indium
D2EHPA (Di(2-ethylhexyl) phosphoric acid)
Application: Effective for separation of indium and zinc.
Characteristics: A phosphoric acid-based solvent that allows selective extraction of metals by adjusting pH.
TBP (Tributyl phosphate)
Applications: Used for the concentration and purification of indium.
Characteristics: Phosphate ester, especially in its ability to extract metal ions from acidic solutions.
Ketone solvents (MIBK: Methyl isobutyl ketone)
Use: Effective extraction of indium in acidic environments.
Characteristics: Commonly used as an organic solvent.
These extractants are usually used in conjunction with acidic solutions for selective extraction from indium-containing sludges and sulfuric acid solutions; D2EHPA is a particularly industrially widespread extractant that allows for efficient separation from zinc and other metals.
Approximate Amount of Compensation
Amount of extractant supplement: about 0.5 to 2 kg per ton of concentrate processed
Circulation utilization: 90-95%.
These extractants are used in multi-stage extraction processes and are recovered and reused after use, but require periodic replenishment as they degrade.
D2EHPA (diethylhexyl phosphate)
Below are the approximate inputs, energy, and waste for producing 1 kg of D2EHPA.
Input power
Power input: 2-4 kWh/kg
(power for heating, stirring, distillation, etc. throughout the process)
Input fuel
Fuel type: natural gas or petroleum-based fuel
Quantity: 1 to 2 kg/kg
CO2 emissions other than combustion
CO2 emissions: 0.5 to 1.2 kg/kg
Amount of change to resources such as soil and water
Water consumption: 50-70 L/kg
(for cooling and process cleaning)
input
Phosphoric acid (H3PO4): 2-3 kg
Used in the esterification reaction of phosphoric acid as the main raw material.
2-Ethyl hexanol: 1 to 1.5 kg
Esterified with phosphoric acid as an alcohol source.
Sulfuric acid: 0.5 to 1.0 kg
Used as a catalyst and removed after the reaction.
Solvent and circulation rate
Organic solvent: toluene or hexane
Amount used: 5-8 kg/kg
Circulation rate: 90-95%.
Waste requiring special treatment
Name: Waste acid (byproduct of sulfuric acid)
Quantity: 0.2-0.5 kg/kg
Requires disposal of waste acid and can be recycled.
Process Overview
esterification reaction
Phosphoric acid and 2-ethyl hexanol react in the presence of a sulfuric acid catalyst.
Separation by solvent
D2EHPA is extracted using an organic solvent such as toluene.
Refining and Distillation
Impurities are removed by distillation to concentrate the product.
Waste Disposal
Recirculate and treat spent sulfuric acid and solvents.
In this process, some of the catalyst sulfuric acid is discarded due to degradation, but much of it is recycled. Organic solvents can also be reused at a high recirculation rate.
Methyl isobutyl ketone (MIBK)
Approximate input energy and substances
Input power
Power input: 1 to 2 kWh/kg
(used for agitation and separation processes during the reaction process)
Input fuel
Fuel type: natural gas or LPG
Quantity: 1.2 to 2 kg/kg
(used in distillation and heating processes)
CO2 emissions other than combustion
CO2 emissions: 0.3-0.8 kg/kg
Amount of change to soil and water
Water consumption: 50-100 L/kg
(for cooling water and cleaning applications)
input
Acetone: 1.5-2 kg
(main raw material for synthesizing methyl isobutyl ketone)
Hydrogen gas (H₂): 0.02-0.05 kg
(required for pressurized reductive reaction)
Catalyst:
Name: Copper-Chromium Catalyst
Amount used: 0.1-0.3 kg/kg
Degradation rate: 2-5% (gradually decreasing while being recycled)
Solvents and circulation rates
Solvent: Hexane
Amount used: 3-5 kg/kg
Circulation rate: 95% or more (reused in distillation)
Waste and its treatment
Name: Waste catalysts and reaction byproducts
Amount: 0.05-0.1 kg/kg
(Waste is disposed of at appropriate treatment facilities.)
Manufacturing Process Overview
Pressure reduction of acetone:
Acetone and hydrogen react in the presence of a copper-chromium catalyst to form methyl isobutyl ketone.
Separation and purification:
Extract the product with hexane solvent to remove impurities.
Distillation and recycling:
MIBK is recovered by distillation and the solvent is used in circulation.
In this process, most catalysts and solvents are reused, but catalysts degrade with each use and must be replaced as needed. In addition, the by-products and waste generated by the reaction must be properly disposed of.
2-Ethyl hexanol (2-EH)
Approximate input energy and substances
Input power
Power input: 1.5-3 kWh/kg
(used for operation of reaction tanks and distillation facilities)
Input fuel
Fuel type: natural gas or LPG
Volume: 0.8-1.5 kg/kg
(used for distillation and heating)
CO2 emissions other than combustion
Emissions: 0.2-0.5 kg/kg
(from reaction and treatment of by-products)
Amount of change to soil and water resources
Water consumption: 30-50 L/kg
(cooling and cleaning applications)
input
Butyraldehyde (C₄H₈O): 1.2-1.5 kg
(main raw material for 2-ethyl hexanol)
Hydrogen gas (H₂): 0.05-0.08 kg
(used in hydrogenation reaction)
Catalyst:
Name: Copper-Chromium Catalysts
Amount used: 0.1-0.3 kg/kg
Deterioration rate: 3-7% (needs to be compensated as appropriate)
Solvents and circulation rates
Solvent: Hexane
Usage: 2-4 kg/kg
Circulation rate: 95% or more (reused after distillation)
Waste requiring treatment
Waste catalyst: 0.05-0.1 kg/kg (discarded after use)
Byproducts: acetic acid and aldehyde byproducts (quantity: 0.1-0.3 kg/kg)
Manufacturing Process Overview
Hydrogenation of butyraldehyde:
Butyraldehyde and hydrogen gas are heated and pressurized in the presence of a copper-chromium catalyst to promote the reaction to produce 2-ethyl hexanol.
Purification of products by distillation:
2-EH is recovered by distillation and by-products are separated.
Catalyst and solvent recycling:
Recovery and replenishment of spent catalyst. Solvents are reused to improve process efficiency.
This process is widely used in the production of 2-ethyl hexanol, which is primarily used in plastic plasticizers, paints, and cosmetic applications. Efficient energy and catalyst management is critical to reduce production costs and environmental impact.
Copper-Chromium Catalysts
Approximate input energy and substances
Input power
Power input: 2-4 kWh/kg
(used for reactor operation, drying and firing processes)
Input fuel
Fuel type: natural gas or heavy oil
Quantity: 1.2 to 2.5 kg/kg
(used for high temperature firing)
CO2 emissions other than combustion
Emissions: 0.3-0.8 kg/kg
(generated from chemical reactions in the process)
Changes in soil and water resources
Water consumption: 50-100 L/kg
(for cleaning and cooling purposes)
input
Copper nitrate (Cu(NO₃)₂): 0.8-1.2 kg
(used as a copper source)
Chromium nitrate (Cr(NO₃)₃): 0.4-0.7 kg
(used as a chromium source)
Alumina (Al₂O₃): 0.3-0.5 kg
(used as catalyst support)
Solvents and process substances
Name: Water
Amount used: 80-120 L/kg
Circulation rate: 85% or more (reuse of cooling water and cleaning water)
waste
Name: Used catalysts
Approximate quantity: 0.05-0.1 kg/kg (periodic disposal due to catalyst degradation)
Name: Acidic liquid waste
Approximate volume: 10-15 L/kg (neutralization treatment required)
Process Overview
Solution preparation: Dissolve copper nitrate and chromium nitrate in an appropriate amount of water.
Coprecipitation process: An alumina support is added to form a catalyst precursor in a coprecipitation reaction.
Drying and calcination: The formed precursors are calcined at 100-500°C to convert them into active catalysts.
Cleaning and regeneration: neutralization and treatment of acidic wastewater by-products during production.
This copper-chromium catalyst is widely used for hydrogenation reactions in the chemical industry. The catalyst degradation rate is 3-5%, requiring periodic replacement with a new catalyst.
butyraldehyde
Approximate input energy and substances
Input power
Power: 0.5 to 1.2 kWh/kg
(used in compression, pumps, and control systems)
Input fuel
Fuel Type: Natural Gas
Amount: 0.6 to 1 kg/kg
(used for heating reaction)
CO2 emissions other than combustion
Emissions: 0.1-0.2 kg/kg
(contained in byproducts and exhaust gas)
Changes in resources such as water, earth, and rocks
Water consumption: 10-20 L/kg
(cooling and cleaning processes)
input
Propylene: 0.8 to 1.0 kg
(used as reaction substrate)
Carbon monoxide (CO): 0.3-0.5 kg
(for oxo reaction)
Hydrogen gas: 0.05-0.1 kg
(maintaining a reduced environment)
Solvents and process substances
Solvent name: Tetrahydrofuran (THF)
Amount used: 0.1-0.2 L/kg
Circulation rate: 80-90%.
Catalyst and degradation rate
Catalyst Name: Rhodium Catalyst
Amount used: 0.002 to 0.005 kg/kg
Deterioration rate: 5-10% (annual renewal required)
Waste requiring treatment
Name: Acidic liquid waste (including catalyst residue)
Amount: 0.1-0.15 kg/kg
(neutralization and disposal required)
Manufacturing Process Overview
Oxo reaction: Butyraldehyde is synthesized by the reaction of propylene and carbon monoxide in the presence of a rhodium catalyst.
Purification and separation: Unwanted byproducts are removed from the product by distillation.
Wastewater treatment: Acidic wastewater generated in the process is neutralized and disposed of safely.
Catalyst regeneration: Recovery and regeneration of rhodium catalyst and replenishment of deteriorated parts.
Butyraldehyde is an important chemical used in the chemical industry to produce plastic additives and plasticizers. The production process requires precise catalyst control, and waste disposal is also an important step.
Copper nitrate (Cu(NO₃)₂)
Approximate input energy and substances
Input power
Electricity: 1.2-1.5 kWh/kg
(used for mixing, heating, dissolving, and pumping)
Input fuel
Fuel Type: Natural Gas
Quantity: 0.5-0.8 kg/kg
(for heating reaction tanks and drying processes)
CO2 emissions other than combustion
Emissions: 0.05-0.1 kg/kg
(as byproducts and reaction gases)
Changes in resources such as water, earth, and rocks
Water consumption: 20-30 L/kg
(dissolution and washing process)
input
Copper concentrate (CuFeS₂, Cu₂S, etc.): 3-4 kg
(assuming a copper content of approximately 25-35%)
Nitric acid: 0.8 to 1.0 kg
(used in the reaction to form copper nitrate)
Solvents and process substances
Solvent name: Water
Amount used: 20-30 L/kg
Circulation rate: 90-95%
(used for process cleaning after reuse)
Catalyst and degradation rate
Catalyst: No catalyst required (acid – based chemical reaction)
Waste requiring treatment
Name: Sulfuric acid waste liquid, iron oxide sludge
Amount: 1.0-1.5 kg/kg
(generated from sulfuric acid compounds and impurities in byproducts)
Manufacturing Process Overview
Copper dissolution: copper concentrate or scrap copper is reacted with nitric acid to produce copper nitrate.
Separation and purification: precipitation of by-products and recovery of copper nitrate from the liquid phase.
Drying: Drying process is performed to obtain crystals from the solution.
Waste Disposal: Acidic effluents and impurities are treated and disposed of in a safe manner.
The purpose of this process is to produce high purity copper nitrate for copper nitrate applications (catalysts, electroplating, agrochemicals, etc.).
Chromium nitrate (Cr(NO₃)₃)
Approximate input energy and substances
Input power
Electricity: 1.5-2.0 kWh/kg
(used for mixing, stirring, heating, and drying processes)
Input fuel
Fuel Type: Natural Gas
Quantity: 0.7-1.0 kg/kg
(heating and drying process)
CO2 emissions other than combustion
Emissions: 0.05-0.08 kg/kg
(by-product of the reaction between chromium oxides and nitric acid)
Changes in resources such as water, earth, and rocks
Water consumption: 15-20 L/kg
(dissolution and process cleaning)
input
Chromium concentrate (containing Cr₂O₃): 3-4 kg
(approx. 30-40% as chromium oxide)
Nitric acid: 0.9-1.1 kg
(used in nitration reaction)
Solvents and process substances
Solvent name: Water
Amount used: 15-20 L/kg
Circulation rate: 85-95%
(washable and reusable)
Catalyst and degradation rate
Catalyst: None (because the reaction proceeds in an acidic reaction)
Waste requiring treatment
Name: Acidic liquid waste, chromium oxide impurities
Amount: 0.8-1.2 kg/kg
(sludge by-product of the refining process)
Manufacturing Process Overview
Dissolution of raw materials: Chromium oxide (Cr₂O₃) is reacted with nitric acid to produce chromium nitrate.
Removal of impurities: Precipitation separation of unwanted by-products.
Concentration and crystallization: The product is concentrated and dried to crystallize chromium nitrate.
Waste treatment: Safe treatment of acidic effluents and impurity sludge.
This process is used to produce chromium nitrate primarily for electroplating and catalytic applications.
Tetrahydrofuran (THF)
Input power
Electricity: 2 to 3 kWh/kg
(used for stirring, reaction control, and refining processes)
Input fuel
Fuel Type: Natural Gas
Quantity: 0.5-0.7 kg/kg
(heating process)
CO₂ emissions other than combustion
Emissions: 0.08-0.12 kg/kg
(depending on chemical reaction and solvent use)
Changes in resources such as earth, rocks, and water
Water consumption: 10-12 L/kg
(cooling and cleaning processes)
input
1,4-Butanediol (raw material): 1.2-1.3 kg
(dehydration cyclization reaction to form THF)
Solvents and process substances
Solvent: Toluene
Amount used: 1 to 2 L/kg
Circulation rate: 90-95% (reused)
Catalyst and degradation rate
Catalyst: Acid catalyst (phosphoric acid-based)
Amount used: 10-15 g/kg
Degradation rate: 5-10%/kg
Waste requiring treatment
Name: Byproducts and unreacted products (polymerized products)
Amount: 0.1-0.2 kg/kg
(residue discharged during separation and purification)
Manufacturing Process Overview
Dehydration cyclization reaction: 1,4-butanediol is dehydrated in the presence of a catalyst to produce THF.
Reaction control and cooling: Reactions are controlled using cooling water.
Distillation and purification: Byproducts are separated to obtain high-purity THF.
Waste disposal: Unreacted materials and byproducts are incinerated or chemically treated.
This process is primarily applied to the production of THF, which is used in solvent and resin production (e.g., polytetrahydrofuran).
rhodium catalyst
Power input (kWh/kg)
80-150 kWh/kg
(required for refining PGM concentrates, separating and reducing rhodium, and sintering)
Fuel input (kg/kg)
Hydrogen gas: 0.15-0.3 kg
(used in reduction process)
Natural gas: 0.1-0.2 kg
(as heat source in refining process)
CO₂ emissions other than combustion (kg/kg)
0.1-0.2 kg
(from acid treatment and byproduct treatment processes)
Change in resources such as soil and water (kg/kg)
Water consumption: 10-15 L
(used in cooling, cleaning and refining processes)
Inputs and Amounts Used
PGM concentrate: 15-20 kg
(rhodium content is about 0.1-0.2% in PGM concentrate)
Sulfuric acid: 2-3 L/kg
(used to dissolve PGMs and remove impurities from concentrates)
Hydrochloric acid: 1 to 2 L/kg
(for dissolving and separating rhodium)
Solvents and process substances
Hydrochloric acid (HCl): 1 to 2 L/kg
Circulation rate: 90-95%
(can be reused)
Organic solvent: toluene or other extraction solvent 0.5 to 1 L/kg
Circulation rate: 85-95%.
Catalyst degradation rate
Degradation rate: 1 to 2%/kg
(loss is small because it can be recycled)
Waste requiring special treatment
Name: Sulfuric acid residue and unreacted metal
Quantity: 0.2-0.5 kg/treatment
Overview of manufacturing process
Acid treatment of PGM concentrates: dissolve with sulfuric acid and hydrochloric acid to dissolve PGM groups (gold, platinum, palladium, etc.).
Individual separation process: Rhodium is selectively extracted using chromatography and solvent extraction methods.
Reduction process: Reduction of rhodium metal from rhodium salts using hydrogen.
Sintering and refining: Sintering at high temperature to produce stable catalysts.
By-product treatment: By-products (residues and acid treatment effluents) are reprocessed and recovered.
The process uses PGM concentrates mined from South Africa and Russia, where rhodium is a small but expensive element in PGMs, and high acid and solvent cycling rates are maintained to limit environmental impact.
Example of correcting the amount of rhodium
Amount of PGM concentrate required:
500-1000 kg (for 0.1-0.2% content)
In this case, the approximate amount of rhodium obtained per ton:
0.1% content → 1 kg in 1 ton
0.2% content → 2 kg per ton
Inputs and energy (modified)
Approximately 500-1000 kg of PGM concentrate is needed to obtain 1 kg of rhodium. The input needs to be modified accordingly.
Example of input modification
PGM concentrate: 500-1000kg
Sulfuric acid: 20-40L
Hydrochloric acid: 10-20L
Hydrogen gas: 0.5-1.0 kg (for reduction)
Energy and Waste
Electricity: 100-150 kWh/kg (used for dissolution, separation, and refining of concentrates)
Waste: Sulfuric acid residue 5-10 kg/1 ton processed
Confirmation of concentrate use in extraction of PGMs
PGMs (platinum group metals), including rhodium, are usually found in concentrates from mines, but rhodium content is so low that large amounts of concentrates must be processed to obtain sufficient amounts. Typically, ores from South Africa and Russia are used for this processing.
chromium concentrates
The process of mining to concentrating chrome concentrates (produced primarily from chromite ore) involves several major stages. These processes are as follows
- mining of ore
It is done by open pit or underground mining.
Mined chromite ore contains many impurities such as soil and rock in addition to chromium-bearing minerals (Cr₂O₃).
- crushing/shredding
The mined ore is crushed in a crusher and reduced to smaller sizes.
A ball mill or rod mill is used to further refine the ore to a particle size suitable for beneficiation.
- beneficiation (physical concentration process)
The following beneficiation techniques are used to obtain chromium concentrates from chromite ores
Gravity Beneficiation: Due to the high specific gravity of chromite, it is separated by gravity using a shaking table or spiral beneficiator.
Magnetic Beneficiation: Chromite is weakly magnetic and uses magnetism to remove non-magnetic components.
Flotation beneficiation: Using chemicals, chromium-bearing minerals are separated from other components.
- conditions for obtaining chrome concentrates
Typically, the chromium content from mined ores is around 10-30% and is enriched to a Cr₂O₃ content of 40-50% or more through a beneficiation process.
After beneficiation, concentrates are mainly used for stainless steel and special alloys.
Inputs and energy throughout the process
Input power (kWh/kg)
2 to 3 kWh/kg: used to operate beneficiation machinery.
Fuel input (kg/kg)
Diesel fuel: 0.2-0.4 kg/kg (for mining and hauling)
- non-combustion CO2 emissions (kg/kg)
0.01 to 0.03 kg/kg (by-product of chemical reaction)
- impact on soil, rocks, and water
Tailings: 3-8 kg/kg waste (soil and rock residue)
Water usage: 10-15 L/kg (often recirculated)
- inputs (process chemicals, etc.)
Floating chemicals: 0.05-0.1 kg/kg (e.g. pine acid)
Coagulant: 0.01-0.05 kg/kg
- solvent with circulation rate
Water used: 80-90% recyclable
- waste
Tailings: 3-5 times the amount of ore mined. Requires disposal.
summary
The process of obtaining chrome concentrates requires a large amount of energy and inputs from ore mining to physical beneficiation to obtain high-purity concentrates. Efficient resource utilization is required at each stage, and the treatment of tailings and wastewater becomes an issue for reducing environmental impact.
The resulting chromium concentrate is then fed into the metallurgical process and becomes an essential material for the manufacture of steel and chemical products.
1,4-Butanediol (BDO)
1,4-Butanediol (BDO) is mainly produced by processes such as the acetylene process and the maleic anhydride process. Below are some general inputs and approximate energy consumption for these processes.
Estimated energy and inputs
Input power
0.5 to 1.0 kWh/kg
(power consumption of pumps, agitators and reaction control system)
Input fuel
Natural gas: 0.3-0.6 kg/kg (for heating)
Hydrogen: 0.01-0.03 kg/kg (used for reduction reactions)
CO₂ emissions other than combustion
0.05-0.1 kg CO₂/kg (by chemical reaction and solvent evaporation)
Impact on soil and water resources
Water usage: 10-15 L/kg (cooling and reaction applications)
Wastewater discharge: 5-8 L/kg (treatment required)
Typical Inputs
Acetylene (for acetylene method): 1.0-1.2 kg/kg
Formaldehyde (for maleic anhydride method): 1.5-1.7 kg/kg
Catalyst:
Copper-based catalyst: 0.01-0.05 kg/kg
Catalyst degradation rate: 5-10%/kg (renewable)
Process Solvents
organic solvent (e.g., tetrahydrofuran):
Usage: 0.2-0.5 kg/kg
Circulation rate: 80-90%.
Waste to be disposed of
Reaction residues and by-products (organic waste): 0.05-0.1 kg/kg
Treatment of wastewater: 5-8 L/kg
summary
The production of 1,4-butanediol (BDO) uses a lot of thermal energy and cooling water in the reaction process. Fuels such as natural gas and hydrogen are used, and catalysts are reused, resulting in a high overall resource recycling rate.
This process makes BDO an important chemical feedstock used in the production of plastics, synthetic fibers, and solvents.
PGM (platinum group metals) concentrate
Input power
15-25 kWh/kg
for driving crushing, grinding and flotation processes
Input fuel
Diesel oil (diesel): 3-4 kg/kg
Used for ore transport and mining operations (open pit and underground mining)
CO₂ emissions other than combustion
0.05-0.1 kg CO₂/kg
Arising from the use of chemicals and disposal of waste
Amount of change to resources such as soil and water
Amount of waste rock: 10-30 tons/kg of concentrate
Water usage: 10-20 m³/kg
Needed for flotation and cleaning process
Typical Inputs
PGM-bearing ores (mainly by-products from nickel and chromium deposits)
Input: 10-20 tons/kg of concentrate
PGM content: 0.1-0.2% (depending on ore)
Lime: 1.5-2.0 kg/kg
Used for pH adjustment
Collectors (usually xanthate): 0.05-0.1 kg/kg
Flotation adsorbs metal particles
Solvents and process substances
Organic extractants (D2EHPA and other phosphate extractants)
No use at this stage (D2EHPA is used in metal extraction from concentrates)
Catalysts
No catalyst (not needed at this stage)
Catalysts are often used in chemical refining after the flotation process
Waste requiring special treatment
Tailings (residual minerals separated by flotation): 15-20 tons/kg
Flotation wastewater: 15-25 m³/kg
Circulated and reused, but final partial disposal is required
Process Overview
Mining
Mining PGM-bearing ores underground or in open pits. PGM content in the ore is 0.1-0.2%.
Crushing and grinding
The ore is ground into small pieces in a crusher to make it suitable for flotation.
Floating election
xanthate collectors and foaming agents are used to screen particles containing PGMs.
concentrate recovery
The concentrate after flotation becomes PGM concentrate and is used in the next refining process.
Conclusion.
The production of PGM concentrates requires a multi-step physical treatment, which requires large amounts of ore, water, and fuel. No solvents such as D2EHPA are used in this stage. The flotation process yields a concentrate containing PGMs, ready for separation of each metal in the next chemical refining process. Tailings and wastewater management are key issues, and these wastes must be reused or treated.
acetylene
Input power
2-3 kWh/kg
Used for water cooling equipment and process control systems.
Input fuel
Calcium carbide: 2.5-3 kg/kg
When using the carbide method, acetylene is produced from calcium carbide.
CO₂ emissions other than combustion
0.1-0.2 kg CO₂/kg
generated from chemical reactions and processing of byproducts.
Amount of change to resources such as soil and water
Water: 5-10 L/kg
Used to break down calcium carbide.
input
Calcium carbide (CaC₂): 2.5 to 3 kg
The main raw material for producing acetylene.
Water: 5-10 L
Acetylene and calcium hydroxide are produced by supplying water.
Solvents and other process substances
Lubricating oil and cooling water: 85-90% circulation rate
Used in water cooling process.
Waste requiring special treatment
Calcium hydroxide (slaked lime): 1.4-1.8 kg/kg
Generated as a byproduct. Disposal or secondary use.
Process Overview
calcium carbide method
The process of reacting calcium carbide (CaC₂) with water to form acetylene (C₂H₂).
Chemical reaction formula:
CaC₂ + 2 H₂O → C₂H₂ + Ca(OH)₂
Cooling and flushing
The acetylene produced is cooled and washed with water to remove oxygen and other impurities.
Gas Recovery
The process of compressing and storing acetylene gas.
summary
Acetylene is produced in a reaction using calcium carbide and water, with calcium hydroxide as a byproduct. Energy consumption is used primarily for process control and cooling, which requires large amounts of calcium carbide as fuel. Calcium hydroxide, a waste product, is being used in secondary applications.
Xanthate
Input power
2-3 kWh/kg
Used for process temperature control and agitator operation.
Input fuel
Natural gas or fuel oil: 1 to 1.5 kg/kg
Needed for reaction tank heating.
CO₂ emissions other than combustion
0.05-0.1 kg CO₂/kg
generated from chemical reaction byproducts and solvent regeneration processes.
Amount of change to resources such as soil and water
Water: 3-5 L/kg
Used in reaction and cooling processes.
input
Alkali metal hydroxides (NaOH): 0.5-0.7 kg
Used for neutralization and reaction acceleration.
Alcohol (e.g. ethanol or methanol): 0.3-0.5 kg
Raw material dissolution and reaction media.
Carbonic acid disulfide (CS₂): 0.8-1.0 kg
The main raw material for xanthate formation.
Solvents and other process substances
Alcoholic solvent (e.g. ethanol): 0.3-0.5 kg (80% circulation)
Used to promote dissolution and reaction, some of which is recovered and reused.
Waste requiring special treatment
Organic solvent waste: 0.1-0.2 kg
Solvent after use.
Sulfur waste: 0.05-0.1 kg
carbon disulfide residues and intermediate products.
Process Overview
Reaction process
Alcohol and carbon disulfide (CS₂) react with sodium hydroxide (NaOH) to produce xanthate.
Neutralization and filtration
After the reaction, neutralize the product and remove impurities by filtration.
Drying and refining
Xanthate is dried and made into the final product.
summary
Xanthates are primarily used as mineral collectors in the flotation process in mines. Production requires carbon disulfide (CS₂) and alkali metal hydroxides. Alcohol is used as a mediator in the reaction, part of which can be reused. As waste, solvent effluents and sulfuric residues are generated and require appropriate treatment.
Calcium carbide (CaC₂)
Input power
3.0 to 3.5 kWh/kg
Used for high temperature processes (2000-2200°C) in electric furnaces.
Input fuel
Coke: 0.5 to 0.8 kg
Used as a carbon source.
CO₂ emissions other than combustion
1.0 to 1.5 kg CO₂/kg
Generated from the thermal decomposition of limestone (CaCO₃).
Amount of change to resources such as soil and water
Limestone: 1.8-2.0 kg
Produces calcium oxide from calcium carbonate (CaCO₃).
input
Limestone (CaCO₃): 1.8 to 2.0 kg
Produces calcium oxide (CaO).
Coke: 0.5 to 0.8 kg
reductants and carbon sources.
Solvents and other process substances
Not used (as it is a dry process).
Waste requiring special treatment
Slag (impurities): 0.3-0.5 kg
Silica and alumina in the raw material are discharged as slag.
Overview of manufacturing process
Preparation Process
Limestone is calcined to produce calcium oxide (CaO).
High temperature reaction (in electric furnace)
CaO and coke are mixed and heated in an electric furnace at 2000-2200°C.
CaO + 3C → CaC₂ + CO
Refining
Calcium carbide is removed from the furnace and impurities are separated as slag.
Process Features
Calcium carbide production is power intensive and relies primarily on high temperature processes in electric furnaces.
Some of the byproduct CO gas may be recovered and utilized, but in many cases the CO₂ emissions are problematic.
This production process is used to meet the demand for calcium carbide in the production of acetylene and as a deoxidizer in steelmaking.
Carbon disulfide (CS₂)
Input power
0.5 to 1.0 kWh/kg
Used for heating processes and process control.
Input fuel
Coke or charcoal: 0.3-0.5 kg
Carbon Sources.
CO₂ emissions other than combustion
0.8 to 1.2 kg CO₂/kg
emitted as a side effect of reactions during the formation of carbon disulfide.
Amount of change to resources such as soil and water
Sulfur: 1.0-1.1 kg
Directly sourced from sulfur resources.
input
Sulfur: 1.0-1.1 kg
Needed as a sulfur source for CS₂.
Coke or charcoal: 0.3-0.5 kg
Carbon Sources.
Solvents and other process substances
Not used
process is done in a high-temperature reaction and requires no solvents.
Waste requiring special treatment
Gaseous impurities (CO₂, CO, etc.): 0.5 to 1.0 kg
reaction byproduct of sulfur and carbon.
Overview of manufacturing process
Preparation Process
Prepared from sulfur and coke/charcoal.
High temperature reaction
Reacts in a furnace at about 700-1000°C.
C + 2S → CS₂
Refining Process
The carbon disulfide produced is purified by gas separation and cooling.
Process Features
Carbon disulfide is used as an industrial solvent, pesticide, and viscosity modifier due to its reactivity.
relies on high temperature processes and the supply of raw materials, sulfur and coke, is critical.
CO₂ and carbon monoxide emissions may require exhaust gas treatment and recovery.
The process operates efficiently in sulfur-rich regions, where carbon disulfide safety controls are critical to the production process.
Thallium (Tl)
Input power
approx. 15-25 kWh/kg
Consumption of thallium in the refining process (electrolytic separation and refinement).
Input fuel
Natural gas: approx. 0.8 to 1.2 kg
Used in the roasting process of ores in heating furnaces.
CO₂ emissions other than combustion
0.5-0.8 kg CO₂/kg
Emissions from chemical processing. Byproduct of reaction with sulfuric acid.
Amount of change to resources such as soil and water
Ore: 50-80 kg
The average content of common thallium-bearing ores is about 0.1-0.2%.
Includes tailings as waste after ore processing.
input
Lead concentrate (or zinc ore): 50-80 kg
Thallium is usually extracted as a byproduct of lead and zinc concentrates.
Sulfuric acid: 2-5 kg
Used for thallium elution process.
Solvents and other process substances
D2EHPA (diethylhexyl phosphate): 0.1-0.3 kg
Used in the extraction process, circulation rate: approx. 90%.
Waste requiring special treatment
Tailings: 40-60 kg
Waste after ore roasting. Requires treatment due to heavy metal content.
Sulfate effluent: 2-3 kg
Effluent that needs to be neutralized.
Overview of manufacturing process
ore processing
Roasting from lead and zinc ores to elute thallium-containing salts.
Solvent extraction
Thallium is purified using extractants such as D2EHPA.
Electrorefining
Thallium is purified to a high purity using electric power.
remarks
Thallium production is difficult to extract from ore and is recovered as a byproduct, so there are no large-scale thallium mines.
Waste management is important and thallium is hazardous and must be handled with care.
This process is often used as a secondary use of recyclable resources, especially in lead and zinc smelting facilities.
zinc (Zn)
Input power
3-5 kWh/kg
Electrolytic zinc method consumes to deposit metallic zinc in an electrolytic cell.
Input fuel
Coal or natural gas: 1 to 2 kg
Used in the roasting process (conversion to zinc oxide). Coal or natural gas is mainly used as fuel, depending on the region.
CO₂ emissions other than combustion
0.8 to 1.2 kg CO₂/kg
emissions in chemical processes such as sulfuric acid reactions.
Amount of change to resources such as soil and water
Zinc ore input: 2-3 kg
Zinc content in common zinc ore (sphalerite: ZnS) is 40-50%.
Name of input and input amount
Zinc concentrate (ZnS): 2-3 kg
Roasting of concentrated zinc ore to convert it into zinc oxide.
Sulfuric acid: 0.5 to 1 kg
Dissolve zinc oxide after roasting and turn zinc into zinc sulfate.
Solvents and other process substances
Organic extractants: 0.1-0.2 kg
Used to separate zinc ions in the refining process. Circulation rate: 90% or more.
Waste requiring special treatment
Waste zinc sulfate solution: 1 to 1.5 kg
Contains unwanted impurities in the electrolysis process.
Tailings after roasting: 1 to 2 kg
sulfur and other metal oxides.
Overview of manufacturing process
Roasting process
Roasting at about 900°C to turn zinc flash ore (ZnS) into zinc oxide (ZnO).
Leaching with sulfuric acid
Zinc oxide after roasting is dissolved in sulfuric acid to produce zinc sulfate solution.
Solvent Extraction and Electrolysis
purified by solvent extraction and zinc metal deposited by electrolysis.
This process consumes large amounts of energy and resources, so there is a need to improve ore content and process efficiency. Another important issue is the treatment of tailings and waste liquids produced during zinc production.
cadmium (Cd)
Because the content of zinc concentrates used in the production of cadmium (Cd) is very low, it is possible that the concentrates used in past responses may not be commensurate with the actual Cd content. The concentration of Cd in a typical zinc concentrate is typically around 0.1-0.3%, and approximately 500-1000 kg of zinc concentrate may be needed to obtain 1 kg of metallic Cd.
Approximate inputs and energy to obtain 1 kg of cadmium
Input power
5-8 kWh/kg
Used in electrolytic and solvent extraction processes.
Input fuel
Natural gas or coal: 1 to 1.5 kg/kg
Used in the roasting process of zinc concentrates.
CO₂ emissions other than combustion
0.4-0.6 kg CO₂/kg
Occurs in the chemical treatment process.
Amount of soil, water, and other resource changes
Ore throughput: 500-1000 kg/kg
Cd is recovered as a byproduct from zinc ore.
Type and amount of input materials used
Zinc concentrate (ZnS): 500-1000 kg
Assumes 0.1-0.3% Cd content.
Sulfuric acid: 1.0-1.5 kg
Dissolve Cd and Zn in the leaching process.
Solvents and other process substances
D2EHPA: 0.05 to 0.1 kg
Metal separation by solvent extraction, circulation rate >90%.
Waste requiring special treatment
Roasting residue: 5-10 kg
Mineral residue left after metal separation.
Sulfuric acid waste: 1 to 2 kg
Appropriate liquid waste treatment is required in accordance with environmental regulations.
Manufacturing Process Overview
Roasting
Zinc ore is heated to convert ZnS to ZnO and separate Cd.
Acid leaching
Treated with sulfuric acid to dissolve zinc and cadmium ions.
Solvent extraction
Selectively extract Cd with solvents such as D2EHPA.
Electrolysis and Final Production
Cd from the extract is reduced to metallic form by electrolysis.
mercury
Process Overview
Mercury (Hg) is usually produced from the sulfide ore **Cinnabar (HgS)**. In the production process, cinnabar ore is heated to vaporize the mercury, which is then cooled and condensed to recover it as liquid mercury. The following guideline is based on this typical process.
Input power
1 to 2 kWh/kg
Used in sintering and mercury cooling and condensing processes.
Input fuel
Coal or natural gas: 0.5 to 1 kg/kg
Used to heat furnaces.
CO₂ emissions other than combustion
0.2-0.5 kg CO₂/kg
Derived from side reactions during refining.
Amount of change in resources such as soil and water
Cinnabar ore: 5-10 kg/kg
Mercury content in ore is about 10-20%.
Names of input materials and approximate input amounts
Cinnabar ore (HgS): 5-10 kg
The main ore from which mercury is refined.
Oxygen: supplied from the atmosphere in the process.
Solvents and other process substances
None (heat treatment process without solvent)
Waste requiring special treatment
Sulfur residue: 2-3 kg
When HgS is calcined, sulfur remains as a byproduct.
Waste gas (SO₂): 0.5 to 1 kg
It is generated by oxidation of sulfides and requires exhaust gas treatment.
Process Details
ore calcination
Cinnabar ore is heated at 850-900°C to vaporize mercury.
Cooling and Condensation
The vaporized mercury is cooled and recovered as liquid mercury.
Byproduct Disposal
Sulfur and SO₂ gases generated during firing are properly treated in accordance with environmental regulations.
summary
The process uses a relatively simple thermal treatment and does not require chemical catalysts or complex solvent treatment. However, because of the high risk of environmental contamination, waste gas treatment and safe waste management are important.
Cinnabar concentrate
Process Overview
Cinnabar (HgS) is primarily used as the primary ore for mercury and is beneficiated after mining using physical and chemical methods. Gravity separation and flotation are used in the beneficiation process. Below is a rough estimate of the inputs and energy required to obtain 1 kg of cinnabar concentrate.
Inputs and energy guidelines
Input power
0.5 to 1.2 kWh/kg
Used in crushing, flotation and gravity separation processes.
Input fuel
Diesel fuel: 0.1 to 0.3 kg/kg
Used in mining and transportation processes.
CO₂ emissions other than combustion
0.05-0.1 kg CO₂/kg
Indirect emissions associated with the use of chemical reagents in the beneficiation process.
Amount of change to resources such as soil and water
Ore (containing HgS): 5-10 kg/kg
Based on mercury content in the raw ore.
Input Item Name and Input Quantity
Ore (cinnabar): 5-10 kg
Amount of ore input to obtain 1 kg of concentrate.
Flotation agent (e.g., xanthate): 0.05-0.1 kg
Used to float cinnabar particles.
Solvents and other process substances
Water: 10-20 kg
For flotation and washing during beneficiation (90% circulation).
Waste requiring special treatment
Tailings (beneficiation residue): 4-9 kg
Contains unused components in the ore.
Used flotation chemical water: 0.1-0.2 kg
Need to process properly.
Process Details
ore mining
Mine ores containing cinnabar from mines.
crushing and beneficiation
crushes the ore and concentrates the cinnabar by gravity separation or flotation.
Flotation and Refining
Remove impurities from concentrates obtained by flotation.
Drying of concentrates
The resulting concentrate is dried to give it its final form.
summary
The cinnabar beneficiation process uses physical separation and chemical flotation. These processes remove impurities from the raw ore to obtain a concentrate with a high mercury content. Environmental considerations require management of spent flotation chemicals and tailings.
copper
- process overview
Copper is produced primarily from copper concentrates, which undergo two stages of smelting and electrolytic refining to obtain high-purity copper. A typical copper concentrate contains about 30% copper, with the remainder being sulfides and other impurities. Below is a rough estimate of each input and energy required to produce 1 kg of copper.
Inputs and energy guidelines
Input power
0.3 to 0.6 kWh/kg
consumed in the electrolytic refining process.
Input fuel
Coke: 0.4-0.5 kg
Used for reduction processes in smelting furnaces.
Heavy oil: 0.05 to 0.1 kg
used in the ore drying and calcination process.
CO₂ emissions other than combustion
0.8 to 1.2 kg CO₂/kg
Formation by sulfur oxidation reactions, etc.
Amount of change to resources such as soil and water
Ore input: 3-4 kg
Considers copper content in concentrate.
Water: 20-30 kg
For cooling and cleaning (water circulation >90%).
Input Item Name and Input Quantity
Copper concentrate: approx. 3-4 kg
Assuming 30% copper content.
Oxygen: 0.1 to 0.2 kg
Used in sulfide oxidation reactions during smelting.
Limestone: 0.05-0.1 kg
fluxing agent for slag generation.
Solvents and other process substances
Sulfuric acid: 0.1-0.15 kg
Used in electrolytic refining solutions.
Water: 20-30 kg used, 90% circulation
Waste requiring special treatment
Slag: 1 to 1.5 kg
Impurities after refining.
Sulfur oxide gas (SO₂): 0.3 to 0.6 kg
It is generated in the smelting process and is often recovered and reused in sulfuric acid plants.
Process Flow
Drying and Firing
Copper concentrate is heated to remove water content and some sulfides.
smelting
Copper sulfide is converted to copper oxide by blowing oxygen in a high-temperature furnace.
converter refining
Copper oxide is reduced with coke to obtain crude copper.
electrolytic-refining
Crude copper is refined in an electrolytic bath to obtain electrolytic copper with a purity of 99.99% or higher.
summary
Electricity and fossil fuels play a major role in this process. Improving the efficiency of electricity used for electrolytic refining and the recovery of sulfur oxides are keys to reducing environmental impact.
Sulfurous acid gas (SO₂) treatment
Sulfurous acid gases are primarily treated using full-gas scrubbing technology or oxidation processes for sulfuric acid production. Typical methods include absorption treatment using lime water or sodium hydroxide (NaOH). Below are the approximate inputs, energy, and byproducts required to treat 1 kg of sulfurous acid gas.
Approximate Inputs and Energy
Input power
0.2 to 0.4 kWh/kg
Used to run scrubbing equipment and pumps.
Input fuel
None (in many cases no additional fuel is needed for the scrubbing process)
CO₂ emissions other than combustion
0.1 to 0.15 kg CO₂/kg
secondary emissions, such as from carbonate decomposition.
Amount of change to resources such as soil and water
Water usage: 5-10 kg
for cleaning and cooling. Circulation rates of 90% or higher are common.
Input Item Name and Input Quantity Guideline
Lime water (Ca(OH)₂): 1.0-1.2 kg
Used to produce CaSO₃ (calcium sulfite).
or sodium hydroxide (NaOH): 0.6 to 0.8 kg
neutralized to produce sodium sulfate (Na₂SO₃).
Solvents and other process substances
Water: 5-10 kg, circulation: 90%.
Waste requiring special treatment
Calcium sulfite (CaSO₃): 1.0-1.2 kg
Byproducts of using limewater.
or sodium sulfate (Na₂SO₃): 0.6 to 0.8 kg
produced when NaOH is used.
Process Flow
Absorption treatment: Sulfurous acid gas is passed through a lime water or sodium hydroxide solution to allow a neutralization reaction to proceed.
Separation of solid by-products: precipitate and separate the CaSO₃ or Na₂SO₃ produced.
Water Reuse: Much of the water used for cleaning is reused after filtration and treatment.
summary
Chemical absorption using limewater or NaOH plays an important role in this treatment method. The byproducts produced may be used for industrial applications (e.g., calcium sulfate can be reused as gypsum). In addition, high water recirculation rates are key to reducing environmental impact.
Calcium hydroxide (Ca(OH)₂)
- manufacturing process overview
Calcium hydroxide is formed by the reaction of quicklime (CaO) and water. This reaction is called “hydration” and is an exothermic reaction that releases energy.
Main reaction:
CaO+H2O→Ca(OH)2CaO+H2O→Ca(OH)2
Inputs and energy guidelines
Input power
0.05 to 0.15 kWh/kg
Used to run agitators and pumps.
Input fuel
Coal and natural gas: 0.2-0.3 kg/kg
Used in the calcination of limestone for the production of quicklime (CaO).
CO₂ emissions other than combustion
0.75-0.8 kg CO₂/kg
Direct emissions from limestone decomposition (CaCO₃ → CaO + CO₂).
Amount of change to resources such as soil and water
Water: 0.3-0.5 kg
Consumed in hydration reactions.
Input Item Name and Input Quantity
Quicklime (CaO): 0.74 kg
Calcined limestone (CaCO₃).
Water (H₂O): 0.26 kg
Consumed in hydration reactions.
Solvents and other process substances
None (no solvents required)
Waste requiring special treatment
CO₂: 0.75-0.8 kg
byproduct from the pyrolysis of limestone.
summary
In the production of 1 kg of calcium hydroxide, the largest energy consuming part is the calcination of limestone to obtain quicklime. This produces carbon dioxide, although some of the CO₂ produced may be used in other chemical processes or construction applications. The production process itself is simple and requires a small amount of electricity, mainly for stirring and running pumps.
This process is used industrially to produce calcium hydroxide through a digestion reaction between CaO and water.
Quicklime (CaO)
- manufacturing process overview
Quicklime (CaO) is obtained by calcining limestone (CaCO₃) at high temperatures. This process is called “calcination” and involves the following reactions
CaCO3→CaO+CO2CaCO3→CaO+CO2
This reaction requires high temperatures of 800-1000°C and consumes large amounts of energy.
Inputs and energy guidelines
Input power
0.2 to 0.5 kWh/kg
Electricity used to operate the firing furnace.
Input fuel
Coal, coke, natural gas: 0.25-0.4 kg/kg
used to maintain high temperatures. The percentage of natural gas use tends to increase.
CO₂ emissions other than combustion
0.78 kg CO₂/kg
CO₂ emitted directly from limestone pyrolysis.
Amount of change to resources such as soil and water
Limestone (CaCO₃): 1.8 to 2.0 kg
required to obtain 1 kg of CaO based on the CaCO₃ content in concentrate.
Input Item Name and Input Quantity Guideline
Limestone (CaCO₃): 1.8 to 2.0 kg
Calcium carbonate produces CaO in the calcination process.
Solvents and other process substances
None
Generally no solvents are used.
Waste requiring special treatment
CO₂ emissions: 0.78 kg
Carbon dioxide is a byproduct of quicklime production.
summary
The production of quicklime is characterized by high energy consumption due to high calcination temperatures and, at the same time, high CO₂ emissions. Improving energy efficiency and CO₂ recovery technologies are future challenges. Limestone input from concentrates is adjusted according to the CaCO₃ content, and approximately 1.8 to 2.0 kg of limestone is required to obtain 1 kg of quicklime.
Silver (Ag)
Manufacturing Process Overview
Silver is usually recovered as a byproduct of lead and zinc ores and copper ores. Silver concentrates obtained from ores are separated into silver by electrolytic refining or chemical refining. The grade of silver concentrates is usually 1-2%, but high silver content is also present in the anode mud of copper and lead electrolytic refining.
Inputs and energy guidelines
Input power
5 to 7 kWh/kg
Power required for the electrolytic refining process.
Input fuel
Coal, natural gas, or fuel oil: 0.4-0.6 kg/kg
Used in ore concentrate and heat treatment.
CO₂ emissions other than combustion
1.5 to 2.0 kg CO₂/kg
byproducts during the processing and refining of silver ores.
Amount of change to resources such as soil and water
Ore volume: 10,000-20,000 kg
Crude ore volume to obtain 1 kg of silver.
Input Item Name and Input Quantity Guideline
Silver concentrate: 50-100 kg (1-2% silver content)
recovered from byproduct ore.
Acids (e.g. nitric acid): 5-10 kg
Used for purification.
Solvents and other process substances
Nitric acid: 5-10 kg (90% circulation)
Used to dissolve impurities and reused.
Waste requiring special treatment
Anode mud: approx. 5-10 kg
Needs to be re-refined due to the presence of other precious metals.
Sulfuric acid waste: 2-3 kg
Needs to be neutralized and treated.
summary
Silver production is usually a byproduct of other metal refining. The process of obtaining concentrate from ore mining is very resource intensive, requiring 10 to 20 tons of ore to obtain 1 kg of silver. Electrolytic refining from by-products is the predominant process, generating anode mud and acid waste as waste products.
Silver concentrate (Ag)
Manufacturing Process Overview
Silver concentrates are produced using flotation and other methods of **silver-bearing ores (lead, zinc ore, and copper ore)**. Because ores contain many components other than silver, efficient ore dressing and flotation are required. This section provides quantitative guidelines for the inputs and energy required to obtain 1 kg of silver concentrate.
Approximate Inputs and Energy
Input power
2 to 5 kWh/kg
Used in beneficiation processes (crushing, flotation, dewatering).
Input fuel
Heavy oil: 0.5 to 1.0 kg/kg
Used in equipment operation and heating processes in beneficiation facilities.
CO₂ emissions other than combustion
0.5 to 1.2 kg CO₂/kg
emissions through the use of flotation chemicals or crushing.
Amount of change to resources such as soil and water
Ore mined: 10-20 tons
Silver concentrates are recovered from ores with silver content of 0.1-0.5%.
Process water usage: 50-100 kg/kg
Recirculated water is also utilized in the flotation and dewatering processes.
Input Item Name and Input Quantity
Silver ore: 10-20 tons
Assumes silver content of 0.1-0.5%.
Flotation agents (e.g., xanthate): 0.2-0.5 kg/kg
agent for separation of silver components from silver ores.
Solvents and other process substances
Xanthate: 0.3 kg (90% circulation)
Used in the ore flotation process and has a high reuse rate.
Waste requiring special treatment
Flotation residue: 10-19 tons/1 kg concentrate
Needs to be processed as ore residue.
Used flotation chemicals: 0.1-0.2 kg
Part of the material is processed, and the parts that are not reusable are discarded.
summary
Silver concentrates are produced by extracting silver components from ores through beneficiation processes such as flotation. 10 to 20 tons of ore must be mined to obtain 1 kg of silver concentrate, and a large amount of water and chemicals are used in the flotation and dewatering processes. A large amount of flotation residue is generated as waste, which must be disposed of properly.
money (written before an amount)
Manufacturing Process Overview
Gold is typically separated from concentrate via flotation or cyanidation processes. The gold content in concentrate is typically around 30-50 ppm (0.003-0.005%), requiring large amounts of ore and energy. Below is a rough estimate of the standard inputs and energy required to produce 1 kg of gold.
Inputs and energy guidelines
Input power
10-20 kWh/kg
Used in grinding, flotation and cyanidation processes and in electrolytic refining.
Input fuel
Fuel oil or diesel: 5-10 kg/kg
For operation of grinding equipment and furnaces.
CO₂ emissions other than combustion
1.5 to 3.0 kg CO₂/kg
Carbon dioxide emissions are mainly due to cyanidation reactions.
Amount of change to resources such as soil and water
Ore mined: 200,000-300,000 kg
Extracts concentrates from ores with 30-50 ppm gold content.
Process water usage: 50-100 m³/kg
Reused in flotation and cyanidation processes.
Input Item Name and Input Quantity Guideline
Gold concentrate: 2,000-3,500 kg
Amount of concentrate reflecting gold content.
Sodium cyanide (NaCN): 0.5-1.0 kg/kg
The main reagents used during gold extraction.
Solvents and other process substances
Sodium cyanide: 0.8 kg (90% circulation)
Used in cyanidation process, some recirculated.
Waste requiring special treatment
Cyanide sludge: 50-100 kg/kg
Waste after cyanidation treatment, requiring environmental treatment.
Tailings (tailings): 180,000-290,000 kg
Needs to be managed as mining residue.
summary
Gold production is an energy- and resource-intensive process, requiring the mining and refining of large quantities of ore to obtain 1 kg of gold. Cyanide recycling and tailings management are important issues in order to reduce the environmental impact.
gold concentrate
Manufacturing Process Overview
The production of gold concentrate is mainly carried out by the following processes
Ore mining: mining of ore by open-pit or underground mining.
Crushing and flotation: mined ore is crushed into smaller pieces and flotation technology is used to separate the gold-bearing concentrates.
Recovery of concentrate: The resulting concentrate is used for further gold refining.
The gold content of gold ores ranges from 0.3 to 10 g/tonne, with concentrates typically grading 30-50 ppm (0.003-0.005%).
Approximate Inputs and Energy
Input power
3-5 kWh/kg
Used to operate crusher and flotation equipment.
Input fuel
Fuel oil or diesel: 0.2-0.5 kg/kg
Fuel for mining and hauling equipment.
CO₂ emissions other than combustion
0.1-0.2 kg CO₂/kg
emissions from milling and flotation processes.
Amount of change to resources such as soil and water
Ore mined: 1,000-5,000 kg/1 kg concentrate
Due to the low gold content, a large amount of ore is required.
Water consumption: 1-3 m³/kg
Process water used in the flotation process.
Input Item Name and Input Quantity Guideline
Gold ore: 1,000-5,000 kg
Depends on gold content.
Limestone (CaO): 0.05-0.1 kg/kg
Used for pH adjustment.
Solvents and other process substances
Xanthogenates: 0.01-0.02 kg (80% circulation)
collectors in flotation.
Waste requiring special treatment
Tailings (tailings): 950-4,950 kg/kg concentrate
The tailings that remain after flotation have a high environmental impact and need to be managed.
Drainage: 1-2 m³/kg
Wastewater after flotation is discharged after treatment.
summary
The production of 1 kg of gold concentrate is very energy and resource intensive. Large quantities of ore must be processed, and environmental considerations are essential. Proper treatment of tailings and wastewater, and recycling of chemicals used in flotation are important.
cyanide disposal
Treatment of cyanide-containing materials, primarily using alkaline chlor-oxidation methods and **iron sulfate (ferrocyanide decomposition)**, is frequently used in gold mining and chemical industry wastewater treatment.
Approximate Inputs and Energy
Input power
1.0 to 2.0 kWh/kg
mixing and stirring equipment and oxidation process equipment operation.
Input fuel
Natural gas: 0.1-0.2 kg/kg
with high temperature decomposition.
CO₂ emissions other than combustion
0.1-0.3 kg CO₂/kg
Occurs as part of the byproducts of chemical reactions.
Amount of change to resources such as soil and water
Amount of water for treatment: 1-5 m³/kg
chemical dilution and wastewater treatment.
Input Item Name and Input Quantity Guideline
Sodium hypochlorite (NaOCl): 0.5 to 1.0 kg
Used as an oxidizer for cyanide.
Iron(II) sulfate (FeSO₄): 0.5 to 1.5 kg
Used to process ferrocyanide.
Solvents and other process substances
Caustic soda (NaOH): 0.3-0.5 kg (80% circulation)
To keep pH alkaline.
Activated carbon: 0.01-0.05 kg (90% circulation)
Used as an adsorbent.
Waste requiring special treatment
Iron oxide precipitate: 0.1-0.3 kg
Ferrocyanide decomposition residue.
Wastewater: 1-3 m³/kg
Wastewater after dilution and chemical treatment.
summary
Oxidizers and pH adjusters are frequently used to treat cyanide-containing materials. In particular, decomposition using oxidants such as sodium hypochlorite and ferrocyanide treatment with iron sulfate are common. Proper management and reuse of wastewater are also important factors to minimize environmental impact.
Iron(II) sulfate
Iron(II) sulfate (FeSO₄) is produced primarily by dissolving iron in sulfuric acid. Below is a rough estimate of inputs, energy, and waste for a typical production process.
Inputs and energy guidelines
Input power
0.1 to 0.2 kWh/kg
Power for stirring, dissolving, and crystallization.
Input fuel
Natural gas: 0.02-0.05 kg/kg
For heating when high temperature processes are required.
CO₂ emissions other than combustion
0.05-0.1 kg CO₂/kg
emissions during iron oxidation reactions and chemical processes.
Amount of change to resources such as soil and water
Water: 1-3 L/kg
for melting and crystallization processes.
Input Item Name and Input Quantity Guideline
Steel scrap or iron powder: 0.3-0.4 kg
oxidized and dissolved to form iron sulfate.
Concentrated sulfuric acid (H₂SO₄): 0.5-0.6 kg
Used for reaction with iron.
Solvents and other process substances
Water: 2-3 L (80-90% circulation)
Used in crystallization process.
Waste requiring special treatment
Iron oxide sludge: 0.05-0.1 kg
Byproducts of insoluble components.
Wastewater: 1 to 2 L/kg
post-chemical-processing
Approximate inputs and energy to produce 1 kg of iron(II) sulfate
The industrial production of iron(II) sulfate (FeSO₄) is accomplished by dissolving iron in sulfuric acid. Below are some guidelines for inputs, energy, and waste disposal for the process.
Inputs and energy guidelines
Input power
0.1 to 0.3 kWh/kg
Consumed by stirring, reaction acceleration, and pumping.
Input fuel
Natural gas: 0.03-0.06 kg/kg
For heating solutions.
CO₂ emissions other than combustion
0.1 to 0.15 kg CO₂/kg
byproducts of the raw material reaction and treatment process.
Amount of change to resources such as soil and water
Water: 1-4 L/kg
Used for cleaning, reaction and dilution of solutions.
Input Item Name and Input Quantity Guideline
Iron powder or steel scrap: 0.4-0.5 kg
raw material for oxidation reactions.
Sulfuric acid (H₂SO₄, 98%): 0.5-0.6 kg
dissolves iron and produces iron sulfate.
Solvents and other process substances
Water: 2-3 L (circulation rate: 80-90%)
Used for dilution and cleaning.
Waste requiring special treatment
Iron oxide sludge: 0.05-0.1 kg
A byproduct of the reaction, requiring treatment.
Wastewater: 1 to 2 L/kg
Effluent that needs to be treated after the reaction.
This process produces iron sulfate on an industrial scale. The treatment of iron oxidation sludge and wastewater is particularly important for environmental management. Natural gas is often used as fuel, and it is recommended that a high water circulation rate be maintained throughout the process.
cobalt (Co)
The industrial production of cobalt is mainly extracted from sulfide and oxide concentrates and uses either hydrometallurgical or pyrometallurgical methods. Below is a rough estimate of energy and inputs based on the hydrometallurgical method.
Approximate Inputs and Energy
Input power
30-40 kWh/kg
required for the electrolytic process and stirred tank operation in wet smelting.
Input fuel
Fuel oil or natural gas: 0.1-0.2 kg/kg
Used in the roasting process.
CO₂ emissions other than combustion
0.15-0.3 kg CO₂/kg
Generated from chemical reactions and wastewater treatment processes.
Amount of change to resources such as soil and water
Water: 3-5 L/kg
Cooling water required for washing and reaction.
Input Item Name and Input Quantity
Cobalt concentrate: 8-12 kg
Assuming a cobalt grade of 10-15% concentrate.
Sulfuric acid: 1.5 to 2.0 kg
Used for acid leaching.
Oxidant (oxygen or hydrogen peroxide): 0.1-0.2 kg
For accelerating oxidation reactions in acid leaching processes.
Solvents and other process substances
Organic extractants (e.g. D2EHPA): 0.05-0.1 kg (90% circulation)
Used for separation and purification of cobalt by solvent extraction.
Waste requiring special treatment
Sulfated wastewater: 1 to 2 L/kg
Wastewater treatment required.
Roasting residue (slag): 1-3 kg
Insoluble waste after metal recovery.
explanation
Cobalt is typically produced as a byproduct of nickel and copper. The wet smelting process is based on leaching with sulfuric acid, and organic solvents such as D2EHPA are used to separate the metals. It is also characterized by the consumption of large amounts of electricity in the electrolytic refining process.
cobalt concentrate
Input power
0.5 to 1.5 kWh/kg
Used for crushing and beneficiation.
Input fuel
Diesel fuel: 0.2 to 0.5 kg/kg
Needed to operate mining equipment and trucks.
CO₂ emissions other than combustion
0.05-0.1 kg CO₂/kg
Occurs from a chemical reaction during the beneficiation process.
Amount of change to resources such as soil and water
Ore (low grade): 10-20 kg
concentrate from ores with a cobalt grade of 1-3%.
Input Item Name and Input Quantity Guideline
Water: 5-10 L/kg
Used for crushing and flotation beneficiation in the beneficiation process.
Collecting agents (e.g., xanthogenates): 0.01-0.05 kg
helps in the flotation beneficiation of mineral particles.
Solvents and other process substances
Foaming agent: 0.005-0.01 kg (80-90% circulation)
Used in the flotation beneficiation process during ore dressing.
Waste requiring special treatment
Tailings (residue after processing): 9-19 kg
Needs to be processed because it contains unwanted components.
Wastewater: 2-5 L
Water treatment is required.
explanation
Cobalt is usually mined as a byproduct from the same veins as nickel and copper. The ore is mined, crushed, and flotation beneficiated into a high-grade concentrate. This process requires water and flotation chemicals for beneficiation and consumes fuel and electricity for mining and processing. The treatment of tailings and wastewater is an important process to reduce environmental impact.
Foaming agent for ore dressing
Input power
1.5 to 3.5 kWh/kg
Used in chemical synthesis and manufacturing processes.
Input fuel
Natural gas: 0.3-0.5 kg/kg
Used to supply heat energy during the process.
CO₂ emissions other than combustion
0.1 to 0.15 kg CO₂/kg
Occurs from chemical reactions during the manufacturing process.
Amount of change to resources such as soil and water
Water: 5-8 L/kg
Used in the manufacturing process and for cooling.
Input Item Name and Input Quantity Guideline
Alcohols (mainly butyl alcohol): 0.7-0.9 kg
as basic raw materials.
Surfactant: 0.05-0.1 kg
To improve foaming.
Solvents and other process substances
Toluene: 0.1-0.15 kg (circulation 80-85%)
Used as a solvent in the manufacturing process.
Waste requiring special treatment
Organic waste liquid: 0.1-0.3 kg
Solvents and byproducts need to be processed.
explanation
Beneficiation foaming agents are chemicals used primarily in the ore flotation process. Typical foaming agents are butyl alcohol as a base material to which a surfactant is added. This improves the flotation efficiency of mineral particles. In production, natural gas is used to supply energy and the solvent is partially recycled and reused. Foam production requires treatment of organic effluents and wastewater to reduce environmental impact.
This process depends on the production of intermediate products in the chemical industry, and the balance between energy and chemicals affects product quality and cost.
nickel (Ni)
Power input (kWh/kg)
8-15 kWh/kg
Mainly used for electrolytic refining or smelting in electric furnaces.
Input fuel
Coal/Coke: 0.5 to 1.0 kg/kg
Used as a heat source and partly as a reducing agent.
Natural gas: 0.3-0.6 kg/kg
Used to supply heat for blast furnaces and drying processes.
CO₂ emissions other than combustion
0.1-0.3 kg CO₂/kg
It is generated from the decomposition reaction of nickel carbonate and nickel sulfate.
Amount of soil, water, and other resource changes
Water: 10-15 L/kg
Used in electrolytic refining, cleaning and cooling processes.
Input Item Name and Input Quantity Guideline
Nickel concentrate: 8-10 kg (10-15% content)
raw material for obtaining nickel metal.
Sulfuric acid: 0.2-0.3 kg
Used to generate electrolyte.
Oxygen: 0.1 to 0.2 kg
Used to promote oxidation in the reduction process.
Solvents and other process substances
Ammonia solution: 0.05-0.1 kg (90% circulation)
used in the nickel leaching process.
Wastes requiring special treatment Name and quantity standard
Slag (including impurities): 1-3 kg
byproduct of nickel smelting.
Waste liquid (containing sulfuric acid): 0.5 to 1.0 kg
Needs to be treated as effluent after electrolysis.
explanation
In nickel production, nickel concentrate obtained from ore mined from mines is used and converted to metallic nickel through the processes of refining and electrolysis. The main heat source is coke or natural gas, and impurities in the concentrate are removed as slag. Electrolytic refining also requires large amounts of electricity and water. Although sulfuric acid and other chemicals used in the electrolysis process are recovered and reused, waste liquid treatment is required.
The entire process is power intensive, and the quality of the concentrate input affects the final energy consumption and quantity of by-products.
Nickel concentrate
Power input (kWh/kg)
1.5 to 3.0 kWh/kg
Used in crushing, grinding and flotation processes.
Input fuel
Diesel fuel: 0.3-0.5 kg/kg
Used to operate mining equipment and transport vehicles.
Electricity auxiliary fuel: 0.1-0.2 kg (coal or natural gas equivalent)
CO₂ emissions other than combustion
0.05-0.1 kg CO₂/kg
Occurs as a side reaction of chemicals in the flotation process.
Amount of soil, water, and other resource changes
Water usage: 5-10 L/kg
Used for flotation processes and dust suppression.
Tailings emissions: 5-7 kg/kg
ore waste after processing.
Input Item Name and Input Quantity
Ore input: 20-40 kg (grade 2-5%)
High-grade nickel sulfide and laterite ores are used.
Lime: 0.05-0.1 kg
Used to adjust pH in flotation.
Copper sulfate: 0.01-0.05 kg
activator during flotation.
Solvents and other process substances
Xanthate (flotation agent): 0.01-0.03 kg (70-80% circulation)
enhances lipophilicity and flotation of minerals.
Waste requiring special treatment Name and quantity guidelines
Tailings (waste rock): 15-20 kg
Waste left over after ore processing.
Flotation residue: 1 to 2 kg
Residue of chemicals left after flotation.
explanation
The production of nickel concentrate requires large quantities of ore. Laterite and sulfide ores are mainly used, and because of the low grade of the ore, impurities are removed through a beneficiation process. Chemicals and pH adjusters are used in the flotation process, but tailings management is important because of the large number of by-products generated.
In tailings processing, it is essential to manage the waste carried out of the mine, which requires reuse of water and reduction of environmental impact. Flotation chemicals are partially
ruthenium (Ru)
Power input (kWh/kg)
5-8 kWh/kg
Used in electrorefining and melting processes.
Input fuel
Natural gas: 1.0-1.5 kg/kg
For heating during refining.
Heavy oil: 0.5 kg/kg
when used for high temperature processing in a furnace.
CO₂ emissions other than combustion
0.2 to 0.5 kg/kg
Emissions from chemical processing.
Amount of soil and water resource change
50-70 kg/kg
by mining and processing to obtain PGM concentrates from raw ore.
Input Item Name and Input Amount
PGM concentrate: 100-150 kg/kg
Ruthenium content around 0.1-0.2%.
Oxygen: 10-15 kg/kg
Required for oxidation reactions.
Solvent/process substance Name, amount used, and recirculation rate
Hydrochloric acid: 20-30 kg/kg
Used for conversion to chloride, circulation rate 50-60%.
D2EHPA (phosphate ester solvent): 2-5 kg/kg
Used for solvent extraction, 90% circulation.
Sodium hydroxide: 5-8 kg/kg
For pH adjustment.
Waste requiring special treatment Name and quantity standard
Sludge (containing heavy metals): 5-10 kg/kg
Byproducts of chemical processing.
Waste acid: 2-3 kg/kg
Needs neutralization and treatment.
Process Overview
Acid leaching of concentrates: PGM concentrates are treated with acid to leach out precious metals.
Solvent Extraction: Ruthenium is separated using solvents such as D2EHPA.
Oxidation/Reduction: Ruthenium oxide is obtained in the oxidation process and the pure metal is recovered by reduction.
Electrolytic refining: Electrolytic processing is used to obtain high-purity ruthenium.
The process involves a high degree of chemical treatment and uses large quantities of acids and solvents, many of which are recycled.
rhodium (Rh)
Power input (kWh/kg)
8-12 kWh/kg
Used for electrolytic refining and melting processes in the refining process.
Fuel input Type and quantity
Natural gas: 1.2-1.8 kg/kg
energy for heating and drying.
Heavy oil: 0.5 to 1.0 kg/kg
Used for sintering and high-temperature refining processes.
CO₂ other than combustion (kg/kg)
0.3-0.5 kg/kg
emissions from chemical reactions and by-product processing.
Resource change in soil, rock, water, etc. (kg/kg)
70-100 kg/kg
Total volume including ore mining, beneficiation, and waste disposal.
Input Item Name and Input Quantity
PGM concentrate: 300-500 kg/kg
Large quantities are required due to rhodium content of 0.1-0.2%.
Oxygen: 15-20 kg/kg
Used in the oxidation process.
Hydrochloric acid: 30-40 kg/kg
Used in conversion to chloride and purification.
Solvent/process substance Name, amount used and circulation rate
D2EHPA (phosphate ester solvent): 3-6 kg/kg
Used for rhodium extraction, circulation is 90-95%.
Nitric acid: 10-15 kg/kg
Used for decomposition and removal of impurities, 50% circulation.
Ammonia water: 5-8 kg/kg
Used in neutralization and double salt formation.
Wastes requiring special treatment Name and quantity standard
Sludge containing heavy metals: 10-20 kg/kg
Occurs in the refining process.
Waste acid: 5-7 kg/kg
Needs neutralization or treatment.
Process Overview
Acid Leaching: PGM concentrates are leached in acidic solutions to dissolve precious metals.
Solvent extraction: rhodium is separated from other PGMs (gold, platinum, etc.) using D2EHPA.
Oxidation-reduction: oxidation in an oxidizing furnace and finally reduction to obtain rhodium metal.
Electrolytic refining: Electrolytic treatment is used to achieve high purity.
The production of rhodium is a costly and environmentally hazardous process. It requires large quantities of acids and solvents, which are recycled and reused whenever possible.
osmium (Os)
Power input (kWh/kg)
10-15 kWh/kg
consumed during the electrolysis and oxidation processes in the refining process.
Fuel input Type and quantity
Natural gas: 1.5 to 2.0 kg/kg
in firing and heating furnaces.
Heavy oil: 0.8 to 1.2 kg/kg
Used for high temperature treatment processes.
CO₂ emissions other than combustion (kg/kg)
0.4-0.6 kg/kg
generated from chemical reactions and by-product separation processes.
Amount of impact on resources such as soil and water (kg/kg)
50-80 kg/kg
By-products and waste from the mining and beneficiation process.
Input Item Name and Input Quantity
PGM concentrate: 400-600 kg/kg
osmium content is about 0.1-0.2% and requires a large amount of concentrates.
Chlorine gas: 5-8 kg/kg
Used in oxidation process to produce osmium tetroxide (OsO₄).
Nitric acid: 20-30 kg/kg
Used in the oxidation process.
Solvent/process substance Name, amount used and circulation rate
D2EHPA (phosphate ester solvent): 3-6 kg/kg
Used in extraction from other precious metals. Circulation rate is 90%.
Organic solvents (e.g. toluene): 4-7 kg/kg
Used for extraction of osmium tetroxide. Circulation rate 85-90%.
Wastes requiring special treatment Name and quantity standard
Heavy metal sludge: 15-25 kg/kg
byproduct of refining.
Waste acid: 10-15 kg/kg
Acid effluent in need of treatment.
Process Overview
Oxidation treatment
PGM concentrates are treated with chlorine gas and nitric acid to produce osmium tetroxide (OsO₄).
Distillation and extraction
Osmium tetroxide is volatile and is separated from other metals by distillation.
Reduction process
The extracted OsO₄ is reduced to obtain pure osmium metal.
Electrorefining
Electrolytic refining further improves purity, if necessary.
The production of osmium involves a unique process that utilizes volatile oxidation, among other things, which requires the use of a large amount of solvents and acids, and the disposal of waste acid and heavy metal sludge is important. The low content of osmium in concentrates requires large amounts of concentrates.
iridium (Ir)
Power input (kWh/kg)
15-20 kWh/kg
consumed in electrolytic and refining processes.
Fuel input Type and quantity
Natural gas: 2.0-3.0 kg/kg
Used for heat treatment and drying furnaces.
Heavy oil: 1.0-1.5 kg/kg
Used as an energy source during high-temperature processing.
CO₂ emissions other than combustion (kg/kg)
0.6-0.8 kg/kg
By-product gases generated during chemical processing.
Amount of change to resources such as soil and water (kg/kg)
50-100 kg/kg
Waste from ore mining and concentrating processes.
Input Item Name and Input Quantity Guideline
PGM concentrate: 400-700 kg/kg
Iridium content of 0.1-0.2% requires large amounts of concentrate.
King’s water: 10-20 kg/kg
Used to dissolve iridium (mixture of hydrochloric and nitric acid).
Chlorine: 2-3 kg/kg
Used for impurity removal.
Solvent/process substance Name, amount used and circulation rate
Organic solvents (e.g. D2EHPA): 3-5 kg/kg
Used to separate iridium from other PGMs. Circulation rate 80-90%.
Acid extractants (e.g. tributyl phosphorus): 5-8 kg/kg
Used in selective extraction of iridium. Circulation rate 90%.
Wastes requiring special treatment Name and quantity standard
Heavy metal sludge: 20-30 kg/kg
byproduct of refining.
Waste acid: 10-15 kg/kg
Need to dispose of used acid solution.
Process Overview
King Water Treatment
PGM concentrates are dissolved in royal water to obtain a solution containing iridium.
Extraction Process
Iridium is separated from other PGMs using organic solvents such as D2EHPA.
Reduction process
Pure iridium metal is obtained from iridium chloride using hydrogen gas or other means.
Electrorefining
Improve purity by electrolytic method, if necessary.
summary
Iridium production requires large amounts of PGM concentrates and energy. Dissolution with royal water and separation with extractants such as D2EHPA are important processes. The catalyst degradation rate is low, and 80-90% of the solvent can be recycled. However, treatment of heavy metal sludge and waste acid is an important issue.
Platinum
Power input (kWh/kg)
25-35 kWh/kg
consumption in electrorefining and melting processes.
Fuel input Type and quantity
Natural gas: 2.0-3.5 kg/kg
Used in high-temperature processing and drying furnaces.
Heavy oil: 1.0 to 2.0 kg/kg
Sources of thermal energy.
CO₂ emissions other than combustion (kg/kg)
0.8 to 1.0 kg/kg
It is generated from chemical reactions and dissolution in wastewater.
Amount of change to resources such as soil and water (kg/kg)
70-150 kg/kg
By-product waste from ore mining and beneficiation.
Input Item Name and Input Quantity Guideline
PGM concentrate: 300-500 kg/kg
Platinum content is about 0.2-0.3%.
Hydrochloric acid: 8-12 kg/kg
Used in the refining process.
Nitric acid: 5-7 kg/kg
Used to generate king water.
Chlorine: 2-4 kg/kg
Used to remove impurities in refining.
Solvent/process substance Name, amount used and circulation rate
D2EHPA or TBP: 3-5 kg/kg
Used for platinum extraction, circulation 85-90%.
Acid extractants: 5-8 kg/kg
Used for selective extraction, 90% circulation.
Wastes requiring special treatment Name and quantity standard
Waste acid: 10-15 kg/kg
Need to treat acid after use.
Heavy metal sludge: 20-30 kg/kg
Byproducts produced in the refining process.
Manufacturing Process Overview
King Water Treatment
PGM concentrates are dissolved in royal water (hydrochloric acid + nitric acid) and platinum is obtained from the solution.
Extraction Process
Separate platinum from other PGMs using D2EHPA or TBP.
Reduction process
Reduction of metallic platinum from platinum chloride using hydrogen.
Electrorefining
If necessary, electrolytic methods are used to improve the purity of platinum.
summary
Platinum production requires large amounts of PGM concentrates, and chemical processing and electrolytic refining are the primary processes; extractants such as D2EHPA and acidic solutions are commonly used, most of which are recycled, but some waste acids and heavy metal sludge require proper treatment.
Typical extractants used for platinum and PGM (platinum group metals) separation are chemicals such as
D2EHPA (Di-2-Ethylhexyl Phosphoric Acid)
Phosphate extractants are used for the selective extraction of metal ions. It is mainly used in the separation of intermediate rare earths and other heavy metals, but is also utilized in PGM separation.
TBP (Tributyl Phosphate)
An organic phosphate ester used in the separation and purification of metals such as platinum and palladium. It is also known for its use in nuclear fuel separation.
cyanide-complex-forming extractants
Trioctylamine (TOA) or amine extractants are used in the process to selectively extract metal complexes such as rhodium and iridium.
These extractants help to selectively separate and purify metals from solution by taking advantage of the ability of each metal to form different oxidation states and complexes. The amount used depends on the amount of metal to be separated and the process efficiency, and generally requires multi-step solvent extraction.
As for the circulation rate, it is often properly regenerated and used, with an efficiency of about 85-95%. However, some extractants may need to be disposed of due to decomposition or contamination.
Tributyl phosphate (TBP)
TBP is industrially produced by reacting phosphoric acid with butanol, often using an acid catalyst (such as sulfuric acid) as a catalyst.
Input and energy guidelines for tributyl phosphate (TBP) production process
Power input:
0.5-1.0 kWh/kg
Fuel input:
Natural gas 0.3-0.5 kg/kg (heating process)
CO2 emissions other than combustion:
0.1-0.2 kg CO2/kg (from raw materials)
Changes to resources such as earth and water:
Water consumption: approx. 10-15 kg/kg (for cooling and reaction)
input
Phosphoric acid (H₃PO₄): 0.35-0.4 kg
Butanol (C₄H₁₀O): 0.75-0.85 kg
Industrially, n-butanol is used.
Catalyst and solvent usage
Sulfuric acid (H₂SO₄): 0.01-0.02 kg (per kg production of catalyst, degradation rate 10-20%/kg production)
Solvent (90% reusable): Used for cleaning associated with reactions, etc.
Waste requiring special treatment
Effluent: 0.1-0.2 kg (waste after acid treatment)
Organic by-products: 0.05-0.1 kg (e.g. unreacted butanol)
TBP is frequently used in metal extraction and nuclear fuel cycles due to its high extraction performance. The production process requires acidic conditions, so sulfuric acid is used as a catalyst, but proper neutralization and effluent treatment is necessary after the reaction. Since many solvents are reused, the recirculation rate is typically 90% or higher.
Butanol (1-butanol)
Butanol is mainly produced from petrochemical feedstocks by oxo-processing or fermentation, but here the values are based on the common oxo-process (production from propylene).
1 kg of butanol Approximate input and energy for manufacturing process
Power input:
0.5-0.8 kWh/kg
Fuel input:
natural gas or hydrogen gas 0.4-0.6 kg/kg
(for reaction heating and synthesis process)
CO2 emissions other than combustion:
0.1-0.3 kg CO2/kg (from chemical reactions and raw materials)
Changes to resources such as soil and water:
use of cooling water: approx. 20-25 kg/kg
Input items and their guidelines
Propylene (C₃H₆): 0.75-0.85 kg
(used as hydrocarbon feedstock)
Carbon monoxide (CO): 0.5-0.6 kg
Hydrogen (H₂): 0.05-0.1 kg
Catalyst and solvent usage and circulation rates
Rhodium or cobalt catalyst: 0.002-0.005 kg (degradation rate 5-10%/kg production)
Solvent (e.g., amine solvent): 0.1-0.2 kg (90% circulation)
Waste requiring special treatment
Unreacted gases and by-products: 0.05-0.1 kg (e.g. ethylene)
Catalyst waste: 0.001-0.002 kg (degraded catalyst)
Process Overview
The oxo process uses propylene and carbon monoxide as feedstock and allows the reaction to proceed in a high-pressure environment to produce butanol. The process uses rhodium and cobalt catalysts, which are reusable but degrade.
Amines are used as solvents to protect the catalyst and waste disposal is required.
Butanol is widely used in the chemical industry and for fuel and solvent applications.
The energy consumption and environmental impact of this process varies slightly depending on the equipment and purity of the raw materials.
Tributylamine (TBA)
Manufacturing Process Overview
Tributylamine (TBA) is produced by an amination reaction using butyl alcohol and ammonia as the main raw materials. The process uses catalysts and optimized reaction conditions to achieve high yields. Control of byproducts is also important.
Details of inputs, energy, and waste
Power input:
approx. 2.5-3.5 kWh/kg (used for process temperature control, agitation, pressure control)
Fuel input:
natural gas and steam (heat supply)
approx. 0.5-0.8 kg/kg
CO₂ other than combustion:.
0.1-0.2 kg/kg (during byproduct treatment and separation processes)
Resource change, such as earth, rocks and water:
water used 3-5 L/kg (used in cooling and cleaning processes)
Input and usage guidelines
Butyl alcohol (main raw material)
1.1-1.2 kg/kg
Ammonia
0.3-0.5 kg/kg
Catalysts (e.g. acidic or basic catalysts)
0.02-0.05 kg/kg (degradation rate 5%/kg product)
Solvents used and circulation rates
Organic solvent (e.g., toluene, hexane)
0.1-0.2 kg/kg (circulation rate 80%)
Estimated amount of waste requiring treatment
Unreacted material, catalyst effluent
0.1 to 0.15 kg/kg
Byproducts (lightweight amines, heavy compounds)
0.05-0.1 kg/kg
Process Notes
Tributylamine production requires highly controlled reaction conditions, with temperature and catalyst selection in particular having a significant impact on product quality. Catalysts are reused multiple times, but need to be replenished periodically as they degrade.
The following substances are typical acidic catalysts used in the production of tributylamine (TBA):
Sulfuric acid (H₂SO₄)
Strongly acidic, widely used common catalyst.
It is also used in amination and dehydration reactions.
paratoluenesulfonic acid (p-TsOH)
Strongly acidic catalyst used in organic synthesis.
It has very high solubility and is used for selective amination reactions.
Hydrochloric acid (HCl)
Easily administered in liquid form, especially applicable to neutralization and amination reactions in reaction systems.
These acidic catalysts promote highly efficient reactions in the manufacturing process, but require post-reaction neutralization and removal processes. In particular, sulfuric acid and paratoluenesulfonic acid are difficult to recycle and require appropriate liquid waste treatment.
manganese (Mn)
Manufacturing process: (1)
Manganese is produced primarily using electrolytic and reduction methods.
In the electrolytic method, high-purity manganese metal is obtained from manganese sulfate solution by electrolysis.
In the reduction process, manganese dioxide (MnO₂) is reduced with coke to obtain manganese metal.
- standard for producing 1 kg of manganese by electrolysis
Power input (kWh/kg)
approx. 6 to 9 kWh
Fuel input Type and quantity (kg)
Coal: 1 to 1.5 kg (used as part of the generation process)
May depend on power supplier
CO₂ emissions other than combustion (kg)
0.5 to 1.0 kg (byproduct of manganese sulfate formation)
Amount of soil, water, and other resources changed (kg)
approx. 5-10 kg water (used in dissolution and washing processes)
Input Item Name and Input Quantity
Manganese sulfate (MnSO₄): 1.6 to 1.8 kg
(for Mn content in concentrate of about 30-50%)
Sulfuric acid: 0.3-0.5 kg (used in dissolution process)
Solvents and other process substances
Name: Water
Usage: 8-12 kg
Circulation rate: 50-70
Wastes requiring special treatment Name and quantity standard
Electrolytic sludge: 0.1-0.3 kg
Waste acid: 0.2-0.5 kg
- standard for producing 1 kg of manganese by the reduction method
Power input (kWh/kg)
4-6 kWh
Fuel input Type and quantity (kg)
Coke: 2-3 kg
CO₂ emissions other than combustion (kg)
approx. 2.5 to 3.5 kg
Amount of soil, water, and other resources changed (kg)
Water: 4-6 kg
Input Item Name and Input Quantity
Manganese dioxide (MnO₂) concentrate: 2.0-2.2 kg
Solvents and other process substances
Name: Water
Usage: 4-8 kg
Circulation rate: 50-70
Wastes requiring special treatment Name and quantity standard
Furnace slag: 1.0-1.5 kg
remarks
The electrolytic method is suitable for producing high-purity manganese, while the reduction method is often used to produce manganese for carbon steel and other alloys.
The reduction method produces more slag, which makes reprocessing and disposal important.
The catalysts and solvents used in each process have a low degradation rate and are mainly limited to acid use in the neutralization process.
manganese concentrate
Manufacturing process: (1)
The main process from the mining of manganese ore to obtaining concentrate involves the following steps
Mining: Mining manganese ore by open pit or underground mining
beneficiation: crushing the coarse ore and obtaining concentrates by magnetic beneficiation or flotation
Dehydrating and drying: removing water from concentrates
Inputs and energy guidelines
Power input (kWh/kg)
0.5 to 0.8 kWh (crushing and beneficiation processes)
Fuel input Type and quantity (kg)
Diesel oil: 0.2 to 0.5 kg (for operation of mining equipment and generators)
CO₂ emissions other than combustion (kg)
0.02 to 0.05 kg (emissions from flotation agents and treatment chemicals)
Amount of soil, water, and other resources changed (kg)
Waste rock and tailings: 5-7 kg
Water used: 8-12 kg (including circulation use)
Input Item Name and Input Quantity
Manganese ore: 2.5-3.5 kg (30-40% manganese content in ore)
Flotation agent (e.g., xanthate): 0.01-0.02 kg
Solvents and other process substances
Water: 8-12 kg
Circulation rate: 60-80%.
Waste requiring special treatment Name and quantity standard
Tailings: 1.5 to 2.5 kg
Flotation waste: 0.3-0.5 kg
remarks
The manganese content in ore varies by region and mine. The average content in ore is about 30-40%.
The beneficiation process primarily uses magnetic beneficiation and flotation. The flotation process requires proper control of chemicals (flotation agent) and flocculants.
During concentrate production, flotation effluent is generated along with waste rock (tailings), which must be treated in accordance with environmental standards.
rhenium (Re)
Manufacturing Process
Rhenium is usually recovered as a by-product from molybdenum concentrates and copper concentrates.
The main processes are as follows
concentrate processing: rhenium oxide is recovered as a byproduct from molybdenum and copper concentrates.
Leaching process: rhenic acid is extracted using acid.
Solvent extraction: selective extraction of rhenium and removal of impurities.
Reduction process: reduction of metallic rhenium from rhenium oxide.
Approximate Inputs and Energy
Power input (kWh/kg)
30-50 kWh (reduction and refining processes)
Fuel input Type and quantity (kg)
Natural gas: 0.5 to 0.8 kg (in reduction furnace)
Diesel oil: 0.2 to 0.5 kg (mine machine operation)
CO₂ emissions other than combustion (kg)
0.1 to 0.3 kg (from use of acids and solvents)
Amount of soil, water, and other resources changed (kg)
Tailings and waste rock: 3-5 kg
Water used: 20-25 kg (including circulation use)
Input Item Name and Input Quantity
Molybdenum concentrate: 20-25 kg (0.02-0.05% rhenium content)
Sulfuric acid: 3-4 kg (for leaching)
Solvents and other process substances
Organic solvent (e.g. tributylphosphate): 0.05-0.1 kg
Circulation rate: 80-90%.
Waste requiring special treatment Name and quantity standard
Acid waste: 0.3-0.5 kg
Impurity sludge: 0.1-0.2 kg
supplementary information
By-product recovery: Rhenium is mainly recovered as a by-product during molybdenum and copper smelting, so the rhenium content of the concentrate itself is very low.
Reduction process: rhenium oxide is commonly converted to metallic rhenium by hydrogen reduction.
Environmental Response: After acid treatment, wastewater treatment is mandatory to increase the percentage of water that can be recycled.
chromium (Cr)
Manufacturing Process
Chromium is obtained primarily from **chromite (chromite: FeCr₂O₄)** and the following processes are common
Reduction process: chromite ore is reduced with coke and other materials in an electric furnace to produce a ferrochrome alloy.
Refining process: High purity chromium is produced by electrolytic refining if necessary.
Inputs and energy guidelines
Power input (kWh/kg)
25-35 kWh (reduction by electric furnace)
Fuel input Type and quantity (kg)
Coke: 1.2 to 1.5 kg (for reduction)
Natural gas: 0.3-0.5 kg (for furnace heating)
CO₂ emissions other than combustion (kg)
0.1 to 0.3 kg (depending on chemical reaction)
Amount of soil, water, and other resources changed (kg)
Tailings and waste rock: 2-4 kg
Water used: 15-20 kg (cooling and dust suppression)
Input Item Name and Input Quantity
Chromium concentrate (40-45% content): 2.5-3 kg
Oxygen: 0.1-0.2 kg (to promote dissolution)
Solvents and other process substances
Sulfuric acid: 0.1-0.3 kg (for impurity removal)
Circulation rate: 80-90%.
Wastes requiring special treatment Name and quantity standard
Slag (chromium oxide residue): 0.5-0.8 kg
Dust waste: 0.1-0.2 kg
supplementary information
Use of chromite ore: to obtain chromium, a chromium content of about 40-45% is required.
Disposal of by-products: some slag from the electric furnace is reused, but the excess needs to be properly treated.
Environmental Responsiveness: Wastewater and slag are strictly controlled, and technology is advancing to increase the efficiency of recycling.
dust treatment
process
Industrially, the following processes are commonly used for dust treatment
Dust collection and filtration: collect dust using fabric filters or electrostatic precipitators.
Wet treatment: Removal by adsorption or precipitation using water or chemicals.
Solidification/stabilization: If it contains hazardous components, stabilize by cement solidification, etc.
Waste disposal: landfill or reuse of residues at disposal sites.
Inputs and energy guidelines
Power input (kWh/kg)
0.3 to 1 kWh (dust collection equipment and pumps in operation)
Fuel input Type and quantity (kg)
Since most operations are electricity-driven, there is little direct fuel input, but natural gas and diesel oil are used in some cases at waste incineration facilities:
Natural gas: 0.05-0.1 kg
Diesel oil: 0.02-0.05 kg
CO₂ emissions other than combustion (kg)
0.01 to 0.02 kg (emissions from wet processing)
Amount of soil, water, and other resources changed (kg)
Water use: 2-5 kg (for wet processing)
Residue: 0.5 to 0.8 kg as residue after solidification
Input Item Name and Input Quantity Guideline
Coagulant: 0.1-0.3 kg (e.g., polyaluminum chloride)
Neutralizer: 0.05-0.1 kg (sodium hydroxide or sodium carbonate)
Solvents and other process substances
Water: 2-5 kg (solvent for wet cleaning)
Circulation rate: 90-95%.
Wastes requiring special treatment Name and quantity standard
Hazardous residues (as the case may be): 0.2 to 0.5 kg (lead and cadmium containing materials requiring stabilization)
Waste filter: 0.01 to 0.02 kg
supplementary information
The treatment process varies depending on the type of dust. In the case of dust from metal smelting plants, extraction of hazardous components is required.
The efficiency of the dust collection system has a significant impact on processing costs. The frequency of bag filter replacement and maintenance must also be considered.
In the case of wet processing, wastewater management is also important, and used water can be recirculated to reduce environmental impact.
molybdenum (Mo)
Manufacturing Process
The production of molybdenum is primarily done by refining from molybdenum concentrate (MoS₂). This process includes the following steps
Roasting process: Molybdenum concentrate (MoS₂) is oxidized to molybdenum trioxide (MoO₃).
Reduction process: MoO₃ is reduced with hydrogen or carbon to obtain metallic molybdenum.
Impurities Removal: In some cases, wet processing is used to remove sulfur and other impurities.
Inputs and energy guidelines
Power input (kWh/kg)
10-20 kWh (for roasting and reduction processes)
Fuel input Type and quantity (kg)
Natural gas: 0.1-0.3 kg (for combustion in roasting furnaces)
Hydrogen: 0.02-0.05 kg (reduction process)
CO₂ emissions other than combustion (kg)
0.02 to 0.05 kg (emissions from sulfur oxides and other byproducts)
Amount of soil, water, and other resources changed (kg)
Water: 1-3 kg (for washing and wet processing)
Input Item Name and Input Quantity Guideline
Molybdenum concentrate (MoS₂): 1.8-2.2 kg (approx. 50-60% molybdenum in concentrate)
Oxygen: 0.5 to 1 kg (roasting process)
Solvents and other process substances
Water: 2-5 kg (circulation: 90-95%)
Wastes requiring special treatment Name and quantity standard
Sulfuric acid (byproduct): 0.2-0.4 kg
Residue: 0.1-0.2 kg (impurities in ore)
supplementary information
In the roasting process, the sulfur in MoS₂ is removed as SO₂ and environmental measures are required.
Hydrogen reduction is the most common method, but carbon reduction is also used in some processes.
Fuel use depends on furnace efficiency and concentrate grade.
Energy management and treatment of emissions throughout the process are key factors affecting costs and environmental impact.
tungsten (W)
Manufacturing Process
Tungsten is mainly produced from scheelite (CaWO₄) and wolframite ((Fe,Mn)WO₄) concentrates. The production process includes the following steps
Alkaline decomposition of concentrate: tungsten is leached to produce sodium tungstate (Na₂WO₄).
Formation of tungstic acid (H₂WO₄) by acid precipitation.
Reduction process: Tungstic acid is reduced with hydrogen gas to obtain metallic tungsten powder.
Produce solid tungsten by high temperature sintering if necessary.
Inputs and energy guidelines
Power input (kWh/kg)
20-30 kWh (reduction and refining process)
Fuel input Type and quantity (kg)
Natural gas: 0.2-0.3 kg (used in drying furnaces)
Hydrogen: 0.05-0.1 kg (for reduction)
CO₂ emissions other than combustion (kg)
0.1 to 0.2 kg (byproducts from chemical reactions)
Amount of soil, water, and other resources changed (kg)
Water: 3-5 kg (washing, solvent extraction, precipitation)
Input Item Name and Input Quantity
Tungsten concentrate (WO₃ basis): 1.5-2.2 kg (70-80% grade concentrate)
Caustic soda (NaOH): 0.1 to 0.3 kg (for alkaline decomposition)
Hydrochloric acid (HCl): 0.2 to 0.5 kg (for acid deposition reaction application)
Solvents and other process substances
Water: 5-8 kg (circulation: 90-95%)
Wastes requiring special treatment Name and quantity standard
Sulfate or hydrochloric acid effluent: 0.5 to 1 kg
Impurity residue: 0.1-0.2 kg (impurities from concentrate)
supplementary information
Hydrogen is mainly used in the reduction process, but could also be used with natural gas to improve energy efficiency.
Disposal of liquid waste as a byproduct is an important process for reducing environmental impact.
To obtain high-purity tungsten, secondary refining and sintering may be required after the reduction process.
Efficient use of resources and waste management are key elements of this process.
Tungsten concentrate
The process for obtaining concentrates from tungsten ores includes mining, beneficiation, and refining. Ores typically processed are scheelite (CaWO₄) and wolframite ((Fe,Mn)WO₄). These ores are processed into concentrates using flotation or gravity beneficiation.
Approximate Inputs and Energy
Power input (kWh/kg)
2-5 kWh (required for beneficiation and flotation processes)
Fuel input Type and quantity (kg)
Diesel and diesel fuel: 0.3-0.5 kg (mining equipment and transport)
Electricity-derived fuels: indirectly consumed at beneficiation facilities
CO₂ emissions other than combustion (kg)
0.02-0.05 kg (indirect emissions from chemical processing and water use)
Amount of soil, water, and other resources changed (kg)
Waste rock (mine waste): 3-5 kg (processing residue)
Water: 8-12 kg (flotation process)
Input Item Name and Input Quantity
Tungsten ore: 1.5 to 3 kg (for ore grades 30-50%)
Flotation chemicals (collectors and foaming agents): 0.05-0.1 kg
Solvents and other process substances
Water: 8-12 kg (circulation: 85-95%)
Wastes requiring special treatment Name and quantity standard
Flotation residue: 2-3 kg
Tailings: 0.5 to 1 kg (landfill disposal or reuse)
Comment
Flotation chemicals include xylene and alcohol-based substances as foaming agents and xanthates as collectors.
Since the use of water resources is directly related to the environmental impact in the vicinity of the mine, it is important to use technology to increase the rate of circulation.
Waste generated during the mining and beneficiation process is an important issue to manage around the mine.
Thus, mining and beneficiation must be closely coordinated to obtain tungsten concentrate, and input and waste management is directly related to production efficiency and environmental friendliness.
niobium (Nb)
Niobium is mainly produced from pyrochlore ore ((Na, Ca)₂Nb₂O₆(F, OH)). Acid and solvent extraction and reduction processes are used to extract and refine niobium from concentrates. The following is a guide to the process used to obtain 1 kg of niobium.
Approximate Inputs and Energy
Power input (kWh/kg)
8-12 kWh (electrolysis and refining processes)
Fuel input Type and quantity (kg)
Natural gas or coke: 0.5-0.8 kg (used in reduction furnaces)
CO₂ emissions other than combustion (kg)
0.1 to 0.3 kg (from chemical reaction)
Amount of soil, water, and other resources changed (kg)
Tailings and slag: 1.5 to 2.5 kg (residue from concentrate)
Water usage: 5-8 kg (acid treatment and cleaning)
Input Item Name and Input Quantity
Niobium concentrate: 1.2-1.5 kg (niobium content in concentrate: 60-70%)
Hydrochloric acid or sulfuric acid: 1 to 1.2 kg (niobium extraction by acid treatment)
Solvents and other process substances
Organic solvent (e.g. D2EHPA): 0.1-0.2 kg (circulation rate: 85-90%)
Wastes requiring special treatment Name and quantity standard
Residue after acid treatment (acid waste): 0.8-1.2 kg
Tailings (unreacted components and impurities): 1.5 kg
Comment
Acid dissolution and solvent extraction play an important role in the niobium production process. This yields niobium of high purity.
In the reduction process, niobium oxide is converted to niobium metal, mainly using aluminum and carbon as reductants.
**D2EHPA (phosphate ester solvent)** is widely used for solvent extraction to separate niobium and tantalum.
The process is designed to be energy efficient and reduce environmental impact, especially in the proper management of waste acid and tailings.
niobium concentrate
Niobium concentrates are obtained primarily from pyrochlore ore and other sources. The process includes mining, crushing, and beneficiation of the ore. Below is a rough estimate of the inputs and energy required to obtain 1 kg of niobium concentrate.
Inputs and energy guidelines
Power input (kWh/kg)
3-5 kWh (grinding, beneficiation, washing)
Fuel input Type and quantity (kg)
Diesel fuel: 0.8 to 1.0 kg (used for mining and transporting ore)
CO₂ emissions other than combustion (kg)
0.2 to 0.5 kg (emissions from chemical reactions during ore beneficiation)
Amount of soil, water, and other resources changed (kg)
Waste tailings: 5-8 kg (unreacted ore and impurities)
Water consumption: 10-15 kg (for beneficiation and washing)
Input Item Name and Input Quantity
Pyrochlore ore: 10-12 kg (8-10% content)
Sodium hydroxide: 0.1-0.2 kg (used for flotation beneficiation)
Solvents and other process substances Name, amount used, circulation rate
Foaming agent for beneficiation (xanthates): 0.05-0.1 kg (circulation: 80-85%)
Wastes requiring special treatment Name and quantity standard
Tailings (containing silica or iron): 5-8 kg
Used beneficiant effluent: 0.5 to 1.0 kg
explanation
In ore mining, open-pit and underground mining are mainly used, and after mining, niobium is enriched by crushing and flotation.
In flotation beneficiation, foaming agents and pH adjusters are utilized to efficiently separate impurities.
Tailings and spent beneficiators require proper waste disposal, and environmental impact is a particular challenge.
The process emphasizes efficient use of energy and resources, and optimal management of the mine leads to increased productivity.
tantalum (Ta)
Tantalum is produced from coltan ore or tantalite. Below is a rough estimate of the inputs and energy required to refine 1 kg of tantalum from concentrate.
Inputs and energy guidelines
Power input (kWh/kg)
15-20 kWh (acid leaching of concentrates, reduction treatment, refining)
Fuel input Type and quantity (kg)
Natural gas: 0.2-0.5 kg (heating in reduction process)
CO₂ emissions other than combustion (kg)
0.8 to 1.2 kg (byproducts during chemical reactions)
Amount of soil, water, and other resources changed (kg)
Water consumption: 8-12 kg (pickling and refining processes)
Waste (impurities, slag): 5-8 kg
Input Item Name and Input Quantity
Tantalum concentrate (tantalite/coltan): 8-10 kg (10-15% content)
Hydrofluoric acid (HF): 0.2-0.5 kg (acid leach)
Sulfuric acid: 0.1-0.2 kg (used in acid treatment process)
Solvents and other process substances Name, amount used, circulation rate
Organic solvent (e.g. methyl isobutyl ketone): 0.1 kg (circulation rate: 90%)
Sodium hydroxide: 0.1-0.2 kg (precipitation of impurities)
Wastes requiring special treatment Name and quantity standard
Acid-treated slag: 3-5 kg
Effluent (residual acid after acid treatment): 1 to 2 kg
explanation
In refining from coltan ore or tantalite ore, the process of acid treating the concentrate to remove impurities is important.
Tantalum oxide is dissolved and converted to tantalum compounds by acid treatment using hydrofluoric acid. It is then reduced to tantalum metal in a reduction furnace.
The reduction process utilizes hydrogen and carbon. Due to the high temperatures, the use of electricity and fuel is essential.
The process is highly energy intensive, and acid handling and waste management are key issues.
Tantalum concentrate
Power input (kWh/kg)
2 to 5 kWh: Used for ore crushing and grinding and beneficiation processes
Fuel input Type and quantity (kg)
Diesel oil: 0.3-0.5 kg (for operation of mining equipment)
Natural gas: 0.1-0.2 kg (drying process)
CO₂ emissions other than combustion (kg)
0.1 to 0.2 kg: generated in the beneficiation process
Amount of soil, water, and other resources changed (kg)
Mined earth and rocks: 8-12 tons (amount of ore processed to obtain 1 kg of concentrate)
Water usage: 20-50 kg (flotation and concentration processes)
Input Item Name and Input Quantity
Tantalite ore: 10-15 kg (content in concentrate: 10-30%)
Sodium hydroxide: 0.1 kg (for precipitation of impurities)
Solvents and other process substances Name, amount used, circulation rate
Organic solvent such as xylene: 0.05 kg (circulation rate: 90%)
Waste requiring special treatment Name and quantity guidelines
Tailings (beneficiation slag): 3-5 kg
Used flotation reagent: 0.1-0.2 kg
explanation
Flotation and gravity beneficiation are the main methods used to obtain tantalum concentrates from tantalite ore and other ores. Due to the low metal content from the ore, a significant amount of ore processing is required.
The amount of earth and rock processed depends on the conditions of the mine and the grade of the ore to be mined. Typically, 10-15 tons of ore may yield 1 kg of tantalum concentrate.
Management of flotation chemicals and water used in the beneficiation process is important, and proper disposal of tailings after treatment is required.
Because the process is energy intensive, it is critical that fuel and power be supplied efficiently at the mining site.
titanium (Ti)
Power input (kWh/kg)
25-35 kWh: Electricity use in metal reduction and refining processes
Fuel input Type and quantity (kg)
Coke: 1 to 2 kg (used in the reduction process)
Natural gas: 0.1-0.2 kg (for drying and heating)
CO₂ emissions other than combustion (kg)
0.3-0.5 kg: CO₂ as byproduct (from reduction process)
Amount of soil, water, and other resources changed (kg)
Mined earth and rocks: 8-10 kg (mined to obtain 1 kg of concentrate)
Water usage: 10-20 kg (cooling and refining)
Input Item Name and Input Quantity
ilmenite concentrate: 4-5 kg (45-50% TiO₂ content)
Chlorine (Cl₂): 0.5 to 1 kg (formation of titanium tetrachloride)
Solvents and other process substances Name, amount used, circulation rate
Mg or Na: 1 to 2 kg (used in the carcina method or reduction process)
Chlorine gas (Cl₂): circulation rate: 80-90%.
Wastes requiring special treatment Name and quantity standard
Waste slag: 1 to 1.5 kg (e.g. iron oxide)
Chlorine compounds in exhaust gases: proper recovery required
explanation
The carcina process is the primary method used in the production of titanium metal. This process involves the reduction of ilmenite concentrate to produce titanium tetrachloride (TiCl₄), which is then reduced to metal. The magnesium reduction process (Kroll process) is primarily used, although sodium is sometimes used as an alternative.
Important Points
The proportion of titanium metal obtained from processing concentrates depends on the grade of the ore, but generally 5 kg of concentrate yields 1 kg of metallic titanium.
Byproducts include chloride slag and spent reductant that must be treated.
This process is energy intensive, consumes high amounts of electricity during the manufacturing phase, and environmental management is critical.
The titanium production process requires multi-step chemical treatment, and ingenuity to increase efficiency, such as chlorine circulation, is essential.
ilmenite concentrate
Power input (kWh/kg)
0.5 to 1.5 kWh: Used to operate beneficiation equipment and pumps
Fuel input Type and quantity (kg)
Diesel fuel: 0.2-0.5 kg (mining equipment and transport)
CO₂ emissions other than combustion (kg)
0.05 to 0.1 kg (flotation beneficiant/byproduct from process)
Amount of soil, water, and other resources changed (kg)
Mined earth and rocks: 5-10 kg (concentrate production from ore with ilmenite grade of 20-30%)
Water usage: 20-50 kg (for beneficiation and slurry processing)
Input Item Name and Input Quantity
Ore (ilmenite ore): 3-5 kg (average content about 20-30% TiO₂)
Flotation beneficiation agent (fatty acid based): 0.02-0.05 kg
Solvents and other process substances Name, amount used, circulation rate
Water: 50 kg used / Circulation rate: 70-90
Wastes requiring special treatment Name and quantity standard
Beneficiated tailings (e.g. iron oxides): 4-6 kg
Wastewater: 10-15 kg (reusable)
Process Description
Mining: ilmenite ore is mined by open-pit or underground mining, with machinery operated by diesel fuel or other means.
Crushing and crushing: Reduces the size of the ore to a particle size suitable for the flotation process.
Flotation Beneficiation: Separation of minerals containing titanium dioxide using fatty acid-based chemicals.
Drying and refining: Concentrate after flotation is dried to concentrate and prepared for transport.
consideration
Water reuse is an important part of the beneficiation process. In many mines, water recycling is used to reduce environmental impact.
Tailings disposal is an environmental challenge and requires proper disposal management.
Energy efficiency is important in the production of ilmenite concentrates, but there are also many byproducts generated from mining to commercialization, which must be managed to reduce the environmental impact.
magnesium (Mg)
Power input (kWh/kg)
30-40 kWh
(Used in fusion electrolysis method. (Electricity used to reduce magnesium chloride in the electrolyzer)
Fuel input Type and quantity (kg)
Natural gas/coke: 0.3-0.6 kg
(used for drying raw materials and heating the electrolyzer)
CO₂ emissions other than combustion (kg)
0.5 to 1.2 kg
(byproduct of electrolyzing magnesium from magnesium chloride)
Amount of soil, water, and other resources changed (kg)
Waste salt sludge: 0.5 to 1 kg
Wastewater: 5-10 kg (used for cooling and cleaning)
Input Item Name and Input Quantity Guideline
Magnesium chloride: 1.2-1.5 kg
(MgCl₂ refined from concentrate)
Calcium compounds (for dehydration): 0.05-0.1 kg
Solvents and other process substances Name, amount used, circulation rate
Calcium chloride (CaCl₂): 0.1-0.2 kg used / circulation rate 70-80% (reused in dewatering process)
Wastes requiring special treatment Name and quantity standard
Electrolytic sludge (salt and impurities): 0.5 to 1 kg
Wastewater: 10 kg (treated and reused)
Manufacturing Process Overview
Purification of raw materials: magnesium chloride obtained from salt lakes and seawater is dehydrated to obtain pure MgCl₂.
electrolytic process:
Using fusion electrolysis, magnesium chloride is reduced in an electrolytic bath. Magnesium is deposited by electrodes and chlorine gas is generated as a byproduct.
Purification and forming: magnesium obtained by electrolysis is cooled and formed into products after forming.
consideration
Energy Efficiency: Efficient energy management is important because the electrolysis process requires large amounts of electricity.
Waste Disposal: Sludge and brine disposal is key to reducing environmental impact.
Circulation and Reuse: Reuse of cooling water and dewatering compounds helps reduce costs and environmental impact.
magnesium chloride
Power input (kWh/kg)
2-5 kWh
(used for heating and dewatering processes)
Fuel input Type and quantity (kg)
Natural gas: 0.2-0.5 kg
(used in furnaces, for dehydration)
CO₂ emissions other than combustion (kg)
0.1 to 0.3 kg
(generated from mineral-derived side reactions)
Amount of soil, water, and other resources changed (kg)
Wastewater: 5-10 kg (post-process brine)
Sludge: 0.1 to 0.3 kg (impurity precipitation)
Input Item Name and Input Quantity
Magnesite concentrate (MgCO₃): 1.5 to 2 kg
(depending on content, magnesium after calcination)
Hydrochloric acid: 0.5-0.8 kg
(for reaction with magnesium)
Solvents and other process substances Name, amount used, circulation rate
Water: 10-20 kg / 70-80% circulation
(used for dissolution and cleaning)
Wastes requiring special treatment Name and quantity standard
Waste brine: 5-8 kg
(treated according to environmental standards)
Solid sludge (impurities): 0.1-0.3 kg
Manufacturing Process Overview
Sourcing raw materials from concentrates:
magnesite (MgCO₃) is pyrolyzed to produce magnesium oxide (MgO).
Reaction with hydrochloric acid:
MgO reacts with hydrochloric acid to yield magnesium chloride (MgCl₂).
Dehydration and concentration: the
resulting MgCl₂ solution is concentrated and dried to pure magnesium chloride.
consideration
Energy Efficiency: Thermal efficiency in the dewatering process has a significant impact on production costs.
Wastewater treatment: used water is circulated, but some needs to be treated as wastewater.
Impurities management: depending on the quality of the concentrate, the amount of sludge produced will also vary.
The process centers on dehydration and reaction with acids, making the solvents used and waste management critical.
Magnesite concentrate
Power input (kWh/kg)
0.5 to 1.5 kWh
(energy for crushing, grinding, and flotation processes)
Fuel input Type and quantity (kg)
Diesel fuel: 0.1 to 0.2 kg
(for heavy mining equipment and hauling)
CO₂ emissions other than combustion (kg)
0.05 to 0.2 kg
(degassing from carbonate in ore)
Amount of soil, water, and other resources changed (kg)
Waste rock and tailings: 10-30 kg (depending on quality)
Water: 5-15 kg (for flotation and cleaning)
Input Item Name and Input Quantity
Magnesite ore: 2-3 kg
(calculated as 30-40% concentrate grade)
Solvents and other process substances Name, amount used, circulation rate
Water: 10-15 kg / 70-80% circulation
(for cleaning and flotation)
Wastes requiring special treatment Name and quantity standard
Tailings: 10-20 kg (residue with impurities)
Wastewater: 2-5 kg (requires proper treatment)
Process Overview
Ore Mining and Transportation
Magnesite ore is mined and moved to the crushing and grinding process.
Concentration by flotation
A flotation process is used to separate magnesite from other minerals. Here, additives and water are used.
Washing and dewatering
After flotation, the concentrate is washed to remove excess impurities.
consideration
Energy efficiency: Energy consumption in the milling and flotation processes is critical.
Tailings management: proper management is necessary because the amount of tailings varies depending on the ore grade.
Use of circulating water: Increasing the rate of water circulation is a cost-saving measure.
Sustainable water management and waste disposal are critical to this process, which requires resource management and reduced environmental impact.
zirconium (Zr)
Power input (kWh/kg)
20-35 kWh/kg
(used for zirconium chloride formation and reduction reaction)
Fuel input Type and quantity (kg)
Natural gas: 0.5 to 1 kg
(heating and reaction maintenance in refineries)
For electrolysis processes, electricity use is the main
CO₂ emissions other than combustion (kg)
1 to 2 kg
(including byproduct emissions)
Amount of soil, water, and other resources changed (kg)
Waste slag: 2-4 kg (acid insoluble residue)
Water consumption: 5-8 kg (for cleaning and cooling applications)
Input Item Name and Input Quantity
Zircon sand: 1.3-1.5 kg
(ZrO₂ content in concentrate is 40-60%)
Chlorine: 1 to 1.5 kg (for zirconium chloride production)
Solvents and other process substances Name, amount used, circulation rate
Chlorine gas: 1.2 kg / circulation rate 90%
(used in chloride reaction, unreacted portion reused)
Water: 5-8 kg / circulation 70-80
Wastes requiring special treatment Name and quantity standard
Slag waste: 2-4 kg
(insoluble residue after acid treatment)
Wastewater: 1-2 kg (acidic effluent)
Process Overview
Zircon Sand Chlorination Process
Zirconium chloride is formed by reaction with chlorine gas at high temperature.
Reduction of Zirconium Chloride
Zirconium is extracted by reduction using magnesium or sodium. The reduction process is mainly carried out in high-temperature environments.
Washing and reprocessing
After purification, metallic zirconium is washed with water to remove impurities.
consideration
Optimize energy consumption: it is important to increase the circulation rate of chlorine gas.
Waste management: slag produced by the chloride reaction needs to be treated.
Choice of reduction method: energy efficiency depends on the choice of electroreduction or metal reduction.
In this process, the recirculated use of chlorine contributes to a reduction in environmental impact and improves cost efficiency.
zircon sand
Power input (kWh/kg)
0.1-0.5 kWh/kg
(drilling, hauling, and operating cleaning equipment)
Fuel input Type and quantity (kg)
Diesel oil: 0.3-0.6 kg
(used in mining equipment and trucking)
CO₂ emissions other than combustion (kg)
0.01 to 0.02 kg
(process derived during mineral processing)
Amount of soil, water, and other resources changed (kg)
Ore/sand to be mined: 10-20 kg
(impurities according to zircon sand content)
Amount of water used: 5-10 kg (for sand washing)
Input Item Name and Input Quantity Guideline
Raw ore (sand containing zircon
sand): 10-20 kg
(5-10% zircon sand content)
Solvents and other process substances Name, amount used, circulation rate
Water: 5-10 kg / circulation rate 80%
(used for cleaning process, partially reused)
Wastes requiring special treatment Name and quantity standard
Slag and residual sand: 9-19 kg
(unwanted sediment and ore impurities)
Process Overview
Mining Mining
open-pit sand and ore to obtain minerals, including zircon sand.
Beneficiation and washing
The drilled ore is washed to remove impurities and extract zircon sand.
Slag and waste sand treatment
Waste sand from the cleaning process is properly treated and some water is reused.
consideration
Resource efficiency: the low content of zircon sand requires large amounts of sediment to be processed.
Environmental impact management: proper disposal of waste sand generated by mining is important.
Energy Consumption: The beneficiation process consumes little energy and is used primarily for washing and transport.
Reuse of wash water is important in this process to reduce the environmental impact. In addition, the management of residual sand and impurities has a significant impact on the efficiency of the mine operation.
hafnium (Hf)
Power input (kWh/kg)
60-80 kWh/kg
(used for reduction and refining processes)
Fuel input Type and quantity (kg)
Natural gas: 0.8 to 1.5 kg
(heating in reduction furnaces, heat source for refining furnaces)
Coke: 0.3-0.5 kg
(to assist in high temperature reduction reactions)
CO₂ emissions other than combustion (kg)
0.05 to 0.1 kg
(from chemical processing and fluoride side reactions)
Amount of soil, water, and other resources changed (kg)
Water consumption: 3-5 kg
(used for cooling and cleaning processes)
By-products other than concentrate: 1-2 kg (slag or waste)
Input Item Name and Input Quantity Guideline
Zirconium concentrate: 5-10 kg
(separated from by-products as hafnium and zirconium coexist)
Hydrofluoric acid (HF): 1 to 2 kg
(used in the hafnium separation process)
Solvents and other process substances Name, amount used, circulation rate
Organic solvent (TBP: tributylphosphate): 0.1-0.3 kg / circulation 90%
(solvent extraction to separate hafnium)
Wastes requiring special treatment Name and quantity standard
Fluoride waste: 0.5 to 1 kg
(needs neutralization treatment)
Slag (impurities including zirconium): 1 to 2 kg
Process Overview
Separation from Zirconium Concentrates
Hafnium is usually extracted as a byproduct of zirconium. After dissolving with hydrofluoric acid (HF), hafnium is separated by solvent extraction using TBP.
Reduction Process
The oxide is reduced to obtain pure hafnium metal. This process uses a high-temperature reduction furnace, electricity, and natural gas.
Final Washing and Cooling
After purification, hafnium is washed with water and cooled to improve purity.
consideration
Main source of hafnium: hafnium is mostly a byproduct of zirconium concentrates.
Management of by-products: disposal of fluoride effluent and slag is important.
Energy Intensity: High temperature reduction process is required, which consumes a lot of electricity and fuel.
Calcium sulfate (CaSO₄)
Power input (kWh/kg)
0.5 to 1.2 kWh/kg
(used for grinding, mixing and drying processes)
Fuel input Type and quantity (kg)
Natural gas: 0.1-0.3 kg
(used in heating processes, drying plaster, etc.)
CO₂ emissions other than combustion (kg)
0.01-0.05 kg
(side reaction from neutralization treatment of acidic wastewater)
Amount of soil, water, and other resources changed (kg)
Water usage: 3-6 kg
(for cleaning and mixing processes)
Input Item Name and Input Quantity Guideline
Limestone (CaCO₃): 0.56 kg
(reacts with sulfuric acid to form CaSO₄)
Sulfuric acid (H₂SO₄): 0.4-0.6 kg
(main reactant)
Solvents and other process substances Name, amount used, circulation rate
Water: usage 3-6 kg / circulation 80%
(process cleaning and hydration reactions)
Wastes requiring special treatment Name and quantity standard
Acidic wastewater: 0.2-0.5 kg (requires neutralization treatment)
Waste gypsum (including impurities): 0.1-0.3 kg
Process Overview
Reaction of limestone with sulfuric acid
Calcium sulfate (CaSO₄) is formed by reacting limestone (CaCO₃) with sulfuric acid (H₂SO₄).
Main chemical reactions: CaCO3+H2SO4→CaSO4+CO2+H2OCaCO3+H2SO4→CaSO4+CO2 +H2O
Drying and grinding
The generated CaSO₄ (dihydrate gypsum or anhydrite) is dried and crushed as needed.
Waste Disposal
Acidic wastewater is generated after the reaction and requires neutralization treatment.
consideration
Main applications: calcium sulfate is used in building materials (gypsum board) and fertilizer.
Environmental Impact: Proper treatment of acidic wastewater is important.
Energy efficiency: drying process requires a lot of energy. Natural gas is the primary fuel source.
Rubidium chloride (RbCl)
Power input (kWh/kg)
10-20 kWh/kg
(used in distillation and crystallization processes in the refining process)
Fuel input Type and quantity (kg)
Natural gas or petroleum-based fuel: 0.5 to 1.0 kg
(used in heating or evaporation processes)
CO₂ emissions other than combustion (kg)
0.01 to 0.03 kg
(emissions from side reactions)
Amount of soil, water, and other resources changed (kg)
Water usage: 5-8 kg (dissolution and cleaning processes)
Input Item Name and Input Quantity
Rubidium-bearing concentrate: 15-20 kg
(Rubidium content in concentrate around 1-1.5%)
Hydrochloric acid (HCl): 0.8-1.2 kg
(main reactant)
Solvents and other process substances Name, amount used, circulation rate
Water: usage 5-8 kg / circulation 80-90%.
Wastes requiring special treatment Name and quantity standard
Acidic liquid waste: 0.5 to 1.0 kg (requires neutralization)
Sludge (impurities): 0.2-0.5 kg
Process Overview
Melting of concentrates
Dissolve ore concentrates containing rubidium in hydrochloric acid to separate impurities.
Extraction and Purification
selectively extracts rubidium from dissolved chloride solutions.
Solvent extraction and ion exchange methods are commonly used.
crystallization
The extracted rubidium chloride is concentrated and crystallized.
Drying and packaging
Finally, the crystals are dried and finished as a powder.
consideration
Main applications: RbCl is used in chemical research and the production of electronic materials.
Problem: Disposal of liquid waste and availability of rubidium-containing ore.
Environmental impact: Proper neutralization and disposal of acidic effluent is important.
The process requires a series of purification and separation techniques to recover rubidium from natural ores with high efficiency.
Rubidium-bearing concentrates
Power input (kWh/kg)
0.3-0.6 kWh/kg
(consumed in mechanical processing for crushing and beneficiation)
Fuel input Type and quantity (kg)
Diesel fuel: 0.2 to 0.4 kg
(used for heavy equipment and transport)
CO₂ emissions other than combustion (kg)
0.01 to 0.02 kg
(emissions from side reactions and beneficiation processes)
Amount of soil, water, and other resources changed (kg)
Raw ore mined: 100-300 kg (0.3-1.0% rubidium content)
Water consumption: 3-5 kg (washing and beneficiation processes)
Input Item Name and Input Quantity
Rubidium-bearing ores (e.g. Lithia pyroxene ore or lepidolite ore): 100-300 kg
(about 1-2% recovery of concentrate from ore)
Flotation chemicals (e.g. xanthate): 0.05-0.1 kg
Solvents and other process substances Name, amount used, circulation rate
Water: 3-5 kg used / 85-90% circulation
Wastes requiring special treatment Name and quantity standard
Tailings (waste): 99-299 kg
(containment required for disposal)
Flotation effluent: 0.1-0.2 kg (chemical treatment required)
Process Overview
Mining
Mining ore in open pit or underground mines.
Crushing and grinding
ore is crushed and grain size is adjusted.
Floating election
Separation of rubidium-bearing minerals using beneficiation chemicals.
Dehydration and drying
The minerals are dehydrated after flotation and finished as concentrate.
consideration
Problem:The low grade of rubidium ore requires large amounts of ore processing.
Environmental Impact: Waste disposal and water recycling use are key issues for environmental management.
Due to the low rubidium content of this process, large volumes of ore must be mined and efficient beneficiation techniques are required.
cesium chloride
Power input (kWh/kg)
1.0 to 2.0 kWh
(evaporation and crystallization process)
Fuel input Type and quantity (kg)
Natural gas: 0.3-0.5 kg
(for heating process)
CO₂ emissions other than combustion (kg)
0.02 to 0.05 kg
(generated by chemical reaction)
Amount of soil, water, and other resources changed (kg)
Water usage: 5-8 kg (purification and cleaning process)
Input Item Name and Input Quantity
Cesium-bearing concentrate: 20-30 kg
(3-5% cesium content in concentrate)
Hydrochloric acid: 2-3 kg (for dissolution and reaction)
Solvents and other process substances Name, amount used, circulation rate
Water: usage 5-8 kg / circulation 85-90%.
Wastes requiring special treatment Name and quantity standard
Insoluble mineral residues: 15-25 kg
(requires reprocessing or containment at a disposal site)
Spent acid solution: 2-3 kg (neutralized and treated effluent)
Process Overview
Dissolution and Extraction
The concentrates containing cesium are dissolved in hydrochloric acid to produce a cesium salt solution.
Impurity Removal
Separate other insoluble components by precipitation or filtration.
Evaporation and crystallization
Formed by heating a saturated solution of cesium chloride and crystallization.
Drying and Packaging
Crystallized cesium chloride is dried to make the final product.
consideration
Neutralization and treatment of effluent is important, and treatment after hydrochloric acid use is part of environmental management.
expensive concentrates, efficient recovery is required.
Cesium-containing concentrates
Power input (kWh/kg)
0.8 to 1.2 kWh
(grinding, flotation and drying processes)
Fuel input Type and quantity (kg)
Diesel fuel: 0.4-0.7 kg
(for mining equipment and transportation)
CO₂ emissions other than combustion (kg)
0.05 to 0.1 kg (gases generated in the treatment process)
Amount of soil, water, and other resources changed (kg)
Ore mined: 50-100 kg
(large amounts of ore are needed due to low cesium content)
Water usage: 8-12 kg (flotation and washing)
Input Item Name and Input Quantity Guideline
Ore (e.g. Pollux ore): 50-100 kg
(Cesium content of the ore is 0.1-0.2%)
Collecting agent (e.g. xanthate): 0.01-0.02 kg
(used for flotation)
Solvents and other process substances Name, amount used, circulation rate
Water: usage 8-12 kg / circulation 85-90%.
Wastes requiring special treatment Name and quantity standard
Tailings (unused ore residue): 40-90 kg
(must be disposed of after stabilization)
Process Overview
Mining
Mining of Pollux ore containing cesium. Open pit or underground mining is common.
crushing and flotation
Mined ore is crushed and flotation separation of cesium-containing minerals using collectants such as xanthates.
Drying and Concentration
Flotation concentrate is dried and processed into concentrate.
consideration
The low content of cesium requires large amounts of ore and increases the burden of residue disposal. In addition, the use of collectors and wastewater treatment are critical to managing environmental impacts.
beryllium (Be)
Power input (kWh/kg)
80-100 kWh (electrolysis process and high temperature reduction process)
Fuel input Type and quantity (kg)
Natural gas: 0.2-0.3 kg
Coal and coke: 0.5-0.8 kg (reduction process)
CO₂ emissions other than combustion (kg)
0.1 to 0.2 kg (byproducts from chemical reaction processes)
Amount of soil, water, and other resources changed (kg)
Soil and tailings: 10-30 kg
Water: 5-10 kg (used throughout the process, required for cleaning and cooling)
Input Item Name and Input Quantity Guideline
Beryllium concentrate (beryl): 7-15 kg (beryllium content approx. 4-5%)
Hydrofluoric acid (HF): 2-3 kg (used to treat Beryl)
Alumina (Al₂O₃): 0.5 to 1 kg (used as coexisting material in the electrolysis process)
Solvents and other process substances Name, amount used, circulation rate
Hydrofluoric acid (HF): usage 2-3 kg / circulation rate 70-80
Sulfuric acid (H₂SO₄): usage 1-2 kg / circulation rate 80
Wastes requiring special treatment Name and quantity standard
Fluoride sludge: 3-5 kg (discarded after chemical treatment)
Unused tailings: 10-20 kg (beryllium ore residue)
Manufacturing Process Overview
Beryl’s Treatment
Beryl ore containing beryllium is decomposed by hydrofluoric acid (HF) to produce beryllium fluoride.
reduction process
Beryllium fluoride is reduced to beryllium metal by electrolysis or high temperature reduction (reacting with carbon or magnesium).
Purification and Solidification
Reduced beryllium is washed and solidified. Forms beryllium metal as a product.
consideration
Beryllium production has high energy costs and requires management of toxic fluorides.
The recycling of sulfuric acid and hydrofluoric acid helps reduce costs and environmental impact, but proper disposal of waste sludge is required.
beryl concentrate
Power input (kWh/kg)
1-2 kWh (beneficiation and grinding processes)
Fuel input Type and quantity (kg)
Diesel oil/diesel fuel: 0.5 to 1 kg (used in mining equipment and transport vehicles)
CO₂ emissions other than combustion (kg)
0.05 to 0.1 kg (by-product from ore processing process)
Amount of soil, water, and other resources changed (kg)
Soil and tailings: 8-15 kg (tailings to be discarded)
Water: 10-20 kg (used in beneficiation process, reusable)
Input Item Name and Input Quantity
Beryllium ore: 15-25 kg (raw ore with 4-5% beryllium content)
Lime (CaO): 1-2 kg (used as pH adjuster in beneficiation process)
Solvents and other process substances Name, amount used, circulation rate
Sulfuric acid (H₂SO₄): usage 0.5-1 kg / circulation rate 80-85
Flotation chemicals: 0.2-0.5 kg used / 70-80% circulation
Wastes requiring special treatment Name and quantity standard
Tailings (tailings): 8-12 kg (needs processing and management)
Flotation effluent: 3-5 kg (requires proper treatment)
Manufacturing Process Overview
Mining and crushing
The raw ore is mined and crushed to a size that is easy to process.
Flotation and beneficiation
Flotation method is used to sort beryl (beryllium-bearing mineral) and separate tailings.
Concentration and Concentration
Beryllium content in concentrate is increased to 4-5% and unwanted substances are removed.
consideration
Sustainable process management is important in beryllium ore beneficiation, where water and chemicals must be reused.
Flotation tailings management and the use of circulating chemicals help reduce costs and environmental impact.
Strontium carbonate (SrCO₃)
Power input (kWh/kg)
1.5 to 3 kWh (concentrate processing and chemical reaction)
Fuel input Type and quantity (kg)
Natural gas: 0.3-0.6 kg (fuel for heating reaction)
Diesel oil: 0.2-0.5 kg (energy assistance for process equipment)
CO₂ emissions other than combustion (kg)
0.4 to 0.8 kg (generated from chemical reaction)
Amount of soil, water, and other resources changed (kg)
Waste rock and tailings: 5-8 kg (unwanted components)
Water: 8-12 kg (for process, recyclable)
Input Item Name and Input Quantity Guideline
Strontium concentrate (Ceres stone): 1.5-2.0 kg (main component to obtain strontium carbonate)
Carbon dioxide (CO₂): 0.5 to 1.0 kg (used for reaction)
Solvents and other process substances Name, amount used, circulation rate
Hydrochloric acid (HCl): usage 0.3-0.5 kg / circulation 85-90%
(remove impurities from concentrate)
Ammonia (NH₃): 0.1-0.3 kg used / 75% circulation
Waste requiring special treatment Name and quantity standard
Salt effluent: 2 to 3 kg (requires water treatment)
Solid waste (tailings): 5-8 kg
Manufacturing Process Overview
Acid treatment of concentrates
Ceres stone (SrSO₄) is treated with hydrochloric acid to produce strontium salts.
carbonation reaction
Strontium salts are passed through carbon dioxide to precipitate strontium carbonate.
Filtration and drying
The strontium carbonate produced is filtered and dried.
consideration
Hydrochloric acid and ammonia can be reused as key process chemicals and require efficient circulation.
requires wastewater treatment and waste management, which affects the overall cost and environmental impact.
Ceres Concentrate (SrSO₄)
Power input (kWh/kg)
0.5 to 1.5 kWh (mining, crushing, beneficiation processes)
Fuel input Type and quantity (kg)
Diesel oil: 0.1-0.3 kg (for drilling and transportation)
Natural gas: 0.1-0.2 kg (drying and heating process)
CO₂ emissions other than combustion (kg)
0.2 to 0.4 kg (side reactions during beneficiation process)
Amount of soil, water, and other resources changed (kg)
Waste rock and tailings: 5-8 kg (impurities from ore)
Water used: 10-15 kg (for washing and flotation processes)
Input Item Name and Input Quantity Guideline
Ore (strontium ore): 2.5-4 kg (average Ceres content 25-35%)
Solvents and other process substances Name, amount used, circulation rate
Collector agent (beneficiation chemicals): 0.05-0.1 kg used / 90% circulation
Defoamer: 0.01-0.05 kg used / circulation 80-85%.
Wastes requiring special treatment Name and quantity standard
Tailings waste: 5-8 kg (to disposal site)
Water treatment effluent: 2-3 kg
Manufacturing Process Overview
ore mining
Strontium ore (including Ceres stone) is mined with excavators and explosives.
crushing and beneficiation
Mined ore is crushed and Ceres stone is sorted by flotation method.
Concentration and Drying
Minerals obtained by flotation are dried to produce Ceres concentrate.
consideration
foaming and collector agents are used in the beneficiation process to increase circulation rates and reduce costs and environmental impact.
Due to the high volume of tailings disposal, waste management and proper water treatment are required.
The amount of water and energy used depends on the grade of the ore and the efficiency of the processing equipment.
Salt Wastewater Treatment
Power input (kWh/kg)
0.3 to 0.8 kWh
needed to drive pumps and provide electrical heating in evaporation and membrane processes.
Fuel input Type and quantity (kg)
Natural gas: 0.05-0.2 kg (used for heating and concentration processes)
Diesel oil: 0.03-0.08 kg (powering waste treatment equipment)
CO₂ emissions other than combustion (kg)
0.1-0.2 kg (CO₂ generation from neutralization process)
Amount of soil, water, and other resources changed (kg)
Water consumption: 2-5 kg (cleaning and reuse)
Generated salt waste: 0.8 to 1.0 kg
Input Item Name and Input Quantity Guideline
Acid (sulfuric acid, hydrochloric acid, etc.): 0.02-0.05 kg (used for pH adjustment)
Alkali (caustic soda, sodium carbonate): 0.02-0.06 kg (neutralization process)
Solvents and other process substances Name, amount used, circulation rate
Flocculant/flocculant (polymer-based flocculant): 0.01-0.02 kg used / circulation 80-90%.
Membrane (RO membrane or nanofilter): degradation rate 1-3%/kg treated
Waste requiring special treatment Name and quantity standard
Concentrated salts: 0.8 to 1.0 kg (can be landfilled or reused)
Sludge: 0.1-0.3 kg (discarded after dewatering)
Process Overview
Pre-treatment: filtering the salt effluent to remove large solids.
pH adjustment: adjust pH with acid or alkali.
Membrane or evaporation treatment: separation with RO membranes or nanofilters, or heat concentration.
Concentrate treatment: dehydrate and solidify the high concentration of salts produced for disposal or reuse.
Water Reuse: Water after treatment is circulated as much as possible.
consideration
Membrane treatment is efficient, but membrane degradation and replacement can be costly.
Evaporative enrichment is energy intensive and may increase fuel use.
The use of chemicals such as coagulants is limited, but the cost and environmental impact of waste disposal is significant.
defoamer
Power input (kWh/kg)
1.5 to 3.0 kWh
Power required for mixing, stirring, and drying processes.
Fuel input Type and quantity (kg)
Natural gas: 0.05-0.1 kg (used for heating and drying process)
Diesel oil: 0.03-0.08 kg (used to power manufacturing equipment)
CO₂ emissions other than combustion (kg)
0.05 to 0.1 kg (generated from chemical reactions and process treatments)
Amount of soil, water, and other resources changed (kg)
Water usage: 2-4 kg (washing and treatment processes)
Input Item Name and Input Quantity Guideline
Silicone fluid: 0.4 to 0.6 kg
Polyether compounds: 0.2-0.4 kg
Emulsifier (e.g. polysorbate): 0.1-0.2 kg
Solvents and other process substances Name, amount used, circulation rate
Alcoholic solvents (e.g., isopropanol): 0.05-0.1 kg used / 90-95% circulation
Water: 3-5 kg / 85% circulation
Wastes requiring special treatment Name and quantity standard
Production by-products (liquid waste containing impurities): 0.1-0.2 kg
Waste solvent: 0.05 to 0.1 kg
Manufacturing Process Overview
Input and mixing of raw materials: silicone fluid, polyether compounds, and emulsifiers are mixed uniformly.
Emulsification process: emulsifier and water are used to produce a stable emulsion.
Solvent addition: Viscosity adjustment with appropriate solvent.
Stirring and heating: Heat as needed to evaporate solvents and water.
Cooling and Finishing: The generated defoamer is cooled and filled in the specified form.
By-product treatment: treatment of waste liquids and solvents generated in the manufacturing process.
supplement
Catalysts are often not required, but small amounts of reaction aids may be used in some production.
The recycling of solvents is an important element for economic and environmental impact reduction.
Appropriate disposal of impurities is required in accordance with laws and regulations.
In the manufacture of defoamers, materials may be distributed differently for specific applications. Quality control is important in each manufacturing process because the product must be designed for each application.
Polyether compounds
Power input (kWh/kg)
1.8 to 3.5 kWh
Used for reaction, mixing, cooling, filtration and drying.
Fuel input Type and quantity (kg)
Natural gas: 0.05 to 0.1 kg (for heating processes)
Diesel oil: 0.02 to 0.05 kg (during production facility operation)
CO₂ emissions other than combustion (kg)
0.1 to 0.3 kg (generated from production reactions)
Amount of soil, water, and other resources changed (kg)
Water usage: 3-6 kg (cooling and cleaning processes)
Input Item Name and Input Quantity
Ethylene oxide (EO): 0.7-0.9 kg
Propylene oxide (PO): 0.1-0.3 kg
Catalyst (KOH): 5-10 g
Solvents and other process substances Name, amount used, circulation rate
Methanol: 0.1-0.2 kg / circulation 80-90%.
Water: 5-8 kg / 85% circulation
Wastes requiring special treatment Name and quantity standard
Waste solvent: 0.05 to 0.1 kg
Effluent containing impurities: 0.1-0.2 kg
Manufacturing Process Overview
Feedstock feed: Ethylene oxide (EO) and propylene oxide (PO) are fed into the reaction tank.
Reaction process: a polymerization reaction is carried out using a catalyst (KOH) to form polyethers.
Neutralization and filtration: After the reaction, neutralize the catalyst with acid and filter to remove it.
Solvent Recovery and Purification: Wash the product with methanol to remove unwanted by-products.
Cooling and Drying: The product is cooled and dried to a specified shape.
Byproduct Disposal: Waste liquids and solvents are collected and properly treated or reused.
supplement
Catalyst degradation rate: 5-10% degradation per kg produced. New catalyst replenishment required.
In the manufacturing process, the ratio of EO and PO can be adjusted according to the application.
Cost reduction and environmental protection through solvent circulation are key factors.
polysorbate
Power input (kWh/kg)
1.5 to 3.0 kWh
Used for reaction processes, cooling, mixing and drying.
Fuel input Type and quantity (kg)
Natural gas: 0.05-0.08 kg (for heating process)
Diesel oil: 0.02-0.05 kg (auxiliary energy for the process)
CO₂ emissions other than combustion (kg)
0.2 to 0.4 kg (by-products of chemical reactions)
Amount of soil, water, and other resources changed (kg)
Water usage: 5-8 kg (cooling and cleaning)
Input Item Name and Input Quantity Guideline
Sorbitan: 0.2-0.4 kg
Ethylene oxide (EO): 0.5-0.7 kg
Fatty acids (typical: stearic acid): 0.3-0.5 kg
Solvents and other process substances Name, amount used, circulation rate
Methanol: 0.1-0.2 kg / circulation 80-85%.
Water: 5-7 kg / 85% circulation
Wastes requiring special treatment Name and quantity standard
Liquid containing waste solvents and impurities: 0.05 to 0.1 kg
Residue of reaction by-products: 0.1-0.2 kg
Manufacturing Process Overview
Esterification reaction: fatty acids and sorbitan react to form basic compounds.
Ethoxylation: ethylene oxide (EO) is used to introduce ethoxyl groups into sorbitan.
Neutralization and washing: Residual reactants are neutralized and washed with methanol.
Filtration and drying: Filter to remove impurities and dry the product.
Quality control: adjust the concentration according to the application.
Catalyst and estimated degradation rate
Acid catalyst (e.g. sulfuric acid): 2-5 g used / 10-15% degradation
Degraded catalysts are regenerated or discarded.
supplementary information
Polysorbate is widely used in surfactants, food additives, and pharmaceuticals.
In the manufacturing process, cost reduction and waste reduction are required by increasing the solvent circulation rate.
Controlling ethoxylation is important in the reaction process and requires adjustment of temperature and reaction time.
propylene oxide
Power input (kWh/kg)
1.8 to 3.2 kWh
Used for precision temperature control and powering pumps, etc.
Fuel input Type and quantity (kg)
Natural gas: 0.1-0.2 kg (for reaction heating)
Hydrogen: 0.02-0.05 kg (for reaction acceleration)
CO₂ emissions other than combustion (kg)
0.5 to 0.8 kg
CO₂ as a byproduct and generated in downstream processes.
Amount of soil, water, and other resources changed (kg)
Water usage: 10-15 kg (cooling and cleaning processes)
Input Item Name and Input Quantity Guideline
Propylene: 0.9 to 1.1 kg
Oxygen: 0.3-0.4 kg
Silver catalyst: 5-10 g
Solvents and other process substances Name, amount used, circulation rate
Methanol: 0.1-0.2 kg / circulation 85-90%.
Water: 10-15 kg / 90% circulation
Wastes requiring special treatment Name and quantity standard
Waste catalyst: 0.5 to 1 g
By-product residue including impurities: 0.2-0.5 kg
Manufacturing Process Overview
Epoxidation reaction: propylene and oxygen react in the presence of a silver catalyst to form propylene oxide.
Cooling and separation: reaction products are cooled and propylene oxide is separated.
Refining: removing residual impurities and byproducts to obtain a high purity product.
Catalyst regeneration: some used catalysts are reused, but the deteriorated ones are discarded.
Catalyst and estimated degradation rate
Silver catalyst: 10-15% degradation/1kg manufactured
Can be regenerated, but some needs to be supplemented with new catalysts.
supplementary information
Propylene oxide is widely used as an intermediate in important compounds such as polyurethanes and glycol ethers.
In manufacturing, circulation of heat and solvents within the process is important for energy efficiency.
sorbitan
Power input (kWh/kg)
1.5 to 2.5 kWh
Used for temperature control and stirring of the reaction system.
Fuel input Type and quantity (kg)
Natural gas: 0.1 to 0.3 kg (for heating process)
CO₂ emissions other than combustion (kg)
0.2 to 0.5 kg
Generated from chemical reaction by-products and refining processes.
Amount of soil, water, and other resources changed (kg)
Water usage: 5-10 kg (for reaction cooling and cleaning applications)
Input Item Name and Input Quantity
Sorbitol: 1.1-1.3 kg (main ingredient)
Acid catalyst (e.g. sulfuric acid): 0.01-0.03 kg
Solvents and other process substances Name, amount used, circulation rate
Methanol: 0.1-0.2 kg / circulation 85-90%.
Wastes requiring special treatment Name and quantity standard
Impurity residue: 0.1-0.2 kg (residue after purification)
Waste catalyst: 0.01 to 0.02 kg
Manufacturing Process Overview
Reaction process:
sorbitol is dehydrated in the presence of an acid catalyst to produce sorbitan. The reaction proceeds at around 120-150°C.
Cooling and Purification:
The reaction products are cooled and byproducts and impurities are removed to obtain high-purity sorbitan.
Catalyst Regeneration and Waste Disposal:
Used acid catalysts are regenerated, but non-reusable portions are discarded.
Catalyst usage and degradation rate
Sulfuric acid catalyst: 5-10% degradation / per kg produced
Partially reused, but new catalyst needs to be added.
supplementary information
Sorbitan is mainly used as a surfactant (raw material for polysorbate products) and is in demand in the food, cosmetic, and pharmaceutical industries.
In-process heat recovery and solvent circulation are important for energy efficiency.
sorbitol (artificial sweetener)
Power input (kWh/kg)
1.8 to 2.5 kWh
Used in pressure control, stirring and heating processes in the hydrogenation process.
Fuel input Type and quantity (kg)
Natural gas: 0.2-0.5 kg (for heating)
CO₂ emissions other than combustion (kg)
0.3 to 0.6 kg (byproducts of the hydrogenation process and water treatment)
Amount of soil, water, and other resources changed (kg)
Water usage: 10-15 kg (used for cooling and solvent processes)
Input Item Name and Input Quantity Guideline
Glucose: 1.05 to 1.1 kg (main ingredient)
Hydrogen: 0.03-0.05 kg (for hydrogenation reaction)
Nickel catalyst: 0.02-0.03 kg (reusable)
Solvents and other process substances Name, amount used, circulation rate
Water: 10-15 kg / circulation 80-90%.
Wastes requiring special treatment Name and quantity standard
Spent catalyst: 0.002 to 0.003 kg
Some is reclaimed, the rest is discarded.
Impurity residue: 0.1-0.2 kg (generated during refining process)
Manufacturing Process Overview
Feed and dissolve raw materials:
Dissolve glucose in water and adjust to appropriate concentration.
Hydrogenation reaction:
Hydrogenation of glucose with a nickel catalyst under high temperature and pressure conditions (130-150°C, approx. 40-60 bar) to produce sorbitol.
Cooling and Purification:
After the reaction, the product is cooled to remove solvent and impurities.
Catalyst regeneration:
used catalysts are collected and reused, some need to be replenished.
Catalyst usage and degradation rate
Nickel catalyst: 10-20% degradation / per kg production
Catalyst is regenerated but needs new replenishment.
supplementary information
Sorbitol is widely used in food, cosmetics, and pharmaceuticals as a sweetener and moisturizer. In the production process, heat recovery and water reuse are important to increase energy efficiency.
Environmental measures: Hydrogen gas control is required for some processes and wastewater treatment facilities will be installed.
Barium sulfate (BaSO₄)
Power input (kWh/kg)
1.0 to 2.0 kWh
Used for mixing, stirring, drying, and filtering.
Fuel input Type and quantity (kg)
Natural gas: 0.1-0.3 kg (for heating in the drying process)
CO₂ emissions other than combustion (kg)
0.05 to 0.1 kg (byproducts from reactions and wastewater treatment)
Amount of soil, water, and other resources changed (kg)
Water consumption: 3-5 kg (used in solution generation and washing process)
Input Item Name and Input Quantity Guideline
Barite concentrate (BaSO₄ content around 90%): 1.1-1.2 kg
Extracts barium components from concentrates.
Sulfuric acid: 0.6-0.8 kg (for sulfation process)
Solvents and other process substances Name, amount used, circulation rate
Water: 3-5 kg / Circulation: 70-80%.
Wastes requiring special treatment Name and quantity standard
Wash water and insoluble residue: 0.2-0.4 kg
Need to treat impurities contained in the product.
Manufacturing Process Overview
Preparation of barite (BaSO₄) concentrate:
The ore is crushed and refined to obtain a high-purity barite concentrate.
Reaction with sulfuric acid:
Barite concentrate is reacted with sulfuric acid to produce concentrated barium sulfate.
Washing and drying:
The reaction products are washed with water and the water is removed in a drying facility.
Impurity Removal and Waste Disposal:
Generated residues and used wash water are disposed of.
Catalyst usage and degradation rate
No catalyst is used, so no degradation occurs.
supplementary information
Barium sulfate is used as an X-ray contrast agent and industrial filler, and purity control is important. In addition, water reuse and wastewater treatment are key environmental measures in the manufacturing process.
scandium (Sc)
Power input (kWh/kg)
15-25 kWh/kg
Used for electrolytic processes and chemical reactions in the refining process.
Fuel input Type and quantity (kg)
Natural gas: 0.3-0.5 kg
Used in drying and heating processes.
CO₂ emissions other than combustion (kg)
0.5 to 1.0 kg
discharged from the byproduct decomposition and effluent treatment processes.
Amount of soil, water, and other resources changed (kg)
Water: 20-30 kg
Used for refining and cleaning, circulation rate needs to be controlled.
Input Item Name and Input Quantity Guideline
Scandium-bearing concentrates (0.1-1% content): 100-200 kg
concentrates often include bastnaesite and thermenite.
Acid (hydrochloric or sulfuric acid): 2-3 kg
For acid dissolution process.
Calcium oxide (CaO): 0.5 to 1.0 kg
Used for removal of impurities and pH adjustment.
Solvents and other process substances Name, amount used, circulation rate
Organic solvent (e.g. tributyl phosphorus): 0.1-0.2 kg / circulation: 80-90%.
Used in solvent extraction processes.
Wastes requiring special treatment Name and quantity standard
Waste acid: 1 to 2 kg
Acid effluent after the dissolution process.
Impurity sludge: 2-3 kg
Generated during metal recovery.
Manufacturing Process Overview
Pre-treatment of concentrates:
scandium-bearing concentrates are crushed and dissolved using acid.
Solvent extraction:
selective extraction of scandium using organic solvents.
Acid Recovery and Reuse:
Acid solutions are reused whenever possible and effluents are properly disposed of.
Concentration and Purification of Scandium:
High purity scandium is recovered by electrolysis or chemical precipitation.
Impurities Treatment and Waste Management:
Impurities are stabilized and landfilled or recycled.
Approximate catalyst usage and degradation rate
Catalyst e.g. tributyl phosphorus.
Usage: 0.1 to 0.2 kg
Degradation rate: 5-10%/kg per production.
supplementary information
Scandium production is an energy-intensive process and solvent extraction technology plays an important role. Environmental impact management and efficient solvent circulation are required. In addition, the recovery of low content from ores such as bastnaesite involves a lot of waste.
Bastnesite concentrate
Power input (kWh/kg)
2 to 5 kWh/kg
required for crushing, flotation beneficiation, and separation processes.
Fuel input Type and quantity (kg)
Diesel fuel: 0.5 to 1.2 kg
Used for mining equipment and haulage vehicles.
CO₂ emissions other than combustion (kg)
0.8 to 1.5 kg
Indirect emissions from beneficiation processes and chemical use.
Amount of soil, water, and other resources changed (kg)
Water: 20-30 kg
For washing and flotation processes required for beneficiation.
Ore residue: 4-6 kg
unwanted ore and sludge.
Input Item Name and Input Quantity
Ore (containing bastnaesite): 30-40 kg
Rare earth content in bastnaesite ore is typically 2-10%.
Sodium hydroxide (NaOH): 0.1-0.3 kg
For pH adjustment in flotation beneficiation.
Sulfuric acid (H₂SO₄): 0.2-0.4 kg
Used to adjust surface activity.
Solvents and other process substances Name, amount used, circulation rate
foaming agent for beneficiation: 0.05-0.1 kg / circulation rate: 80
enhance the effectiveness of flotation.
Flotation agent (e.g., xanthate): 0.01-0.05 kg / circulation: 70-85%.
Wastes requiring special treatment Name and quantity standard
Tailings (slag and tailings): 20-30 kg
Ore residue after beneficiation is disposed of or reused.
Wastewater: 5-10 kg
Must be properly treated after chemical treatment.
Manufacturing Process Overview
Mining and crushing
ores:
Mining ores and crushing them into smaller pieces for concentrate acquisition.
Flotation beneficiation:
separation of bastnaesite from the ore using foaming agents and xanthates.
Washing and drying:
Bastnesite obtained by flotation is washed and dried to concentrate.
Residue and wastewater treatment:
Unnecessary ore residues and wastewater are treated according to environmental standards.
Approximate catalyst usage and degradation rate
Catalyst example: xanthate flotation agent.
Usage: 0.05 kg/ton ore processing.
Degradation rate: 15-20%/new input required per treatment.
supplementary information
Bastnesite is an important source of light rare earths and is commonly separated by flotation technology. Treatment of wastewater and tailings is important to reduce environmental impact, and the reuse of chemicals used is also required.
radium (Ra)
Power input (kWh/kg)
300-500 kWh/kg
Power consumption in the refining and separation treatment processes.
Fuel input Type and quantity (kg)
Natural gas/coal: 1.5 to 2.0 kg
Used for heating processes in furnaces.
CO₂ emissions other than combustion (kg)
5-10 kg
Indirect emissions from sulfuric acid and solvent reactions.
Amount of soil, water, and other resources changed (kg)
Water: 20-30 kg
cooling, solvent treatment, and chemical reaction applications.
Tailings and sludge: 50-100 kg
including residues and radioactive by-products.
Input Item Name and Input Quantity Guideline
Uranium ore concentrate: 500-1000 kg
Radium content is so low that large amounts of uranium concentrate are required.
(Radium content in uranium ore is about 0.0001%)
Sulfuric acid (H₂SO₄): 5-10 kg
Used to elute radium.
Nitric acid (HNO₃): 2-5 kg
Used as an oxidant to accelerate reactions.
Solvents and other process substances Name, amount used, circulation rate
Solvent: diethyl ether: 0.5-1.0 kg / circulation: 90
Used for solvent extraction of radium.
Barium salt: 2-5 kg
facilitates radium precipitation.
Wastes requiring special treatment Name and quantity standard
Radioactive sludge: 10-20 kg
Residues containing uranium and thorium.
Wastewater: 30-50 kg
Residual liquid after solvent or acid treatment.
Manufacturing Process Overview
Processing of concentrate
Uranium ore concentrate is crushed and acid (sulfuric or nitric acid) is used to elute radium.
Precipitation
Precipitate radium as barium radium sulfate by adding barium salt.
Solvent Extraction
Purification of radium using diethyl ether.
Purification and Concentration
Radium is further concentrated, extracted in high purity form and dried.
Waste Disposal
Radioactive sludge and wastewater require specialized radioactive waste disposal.
Approximate catalyst usage and degradation rate
Catalysts: no specific acidic catalysts are used, mainly chemical reactants are important.
Degradation rate: solvent (diethyl ether) circulation rate is about 90%.
supplementary information
The production of radium requires large amounts of processing from very low concentrations of ore, and its high environmental impact requires strict control.
Uranium ore concentrate
Power input (kWh/kg)
0.5 to 1.0 kWh/kg
Used for mining equipment operation, ore crushing, and initial processing steps.
Fuel input Type and quantity (kg)
Diesel fuel: 0.8 to 1.5 kg
Used to operate mining trucks and heavy equipment.
CO₂ emissions other than combustion (kg)
0.02 to 0.1 kg
byproducts of reactions with sulfuric acid and carbonates.
Amount of soil, water, and other resources changed (kg)
Soil and rock: 100-200 kg
The uranium ore grade is about 0.1%, so significant soil removal is required.
Water: 20-50 kg
Used in ore processing and crushing processes.
Input Item Name and Input Quantity Guideline
Uranium ore: 100-150 kg
An ore with a uranium content of 0.1-0.5%.
Sulfuric acid (H₂SO₄): 0.2 to 1.0 kg
Used for uranium extraction by dissolution reaction.
Solvents and other process substances Name, amount used, circulation rate
Organic solvent (tributylphosphate): 0.1-0.3 kg / circulation: 90-95%.
Used in the solvent extraction process of uranium.
Wastes requiring special treatment Name and quantity standard
Tailings: 98-140 kg
Contains trace amounts of radioactive materials and requires proper storage.
Acid waste: 10-20 kg
Effluent after acid treatment. Neutralization treatment is required.
Process Overview
Ore Mining and Crushing
Uranium ore is mined and the grain size is reduced in a crusher.
Leaching process The
uranium is dissolved by adding sulfuric acid to the crushed ore to extract uranium ions from the solution.
Solvent Extraction
Separate uranium in solution using tributylphosphate.
Concentration and precipitation
Uranium compounds are concentrated and precipitated as uranium oxide (yellowcake).
Waste Disposal
After processing, tailings and waste acid are treated and stored according to strict standards.
Approximate catalyst usage and degradation rate
Catalyst: No specific catalyst is used, mainly acid and solvent reactions.
Degradation rate: 90-95% for tributylphosphate circulation.
supplementary information
In the processing of uranium ore, tailings and acid effluents are strictly controlled due to their high environmental impact. Fuel efficiency is also an important factor in mine operations, as mining equipment uses a large amount of fuel.
Long-term isolation treatment of radioactive sludge (more than 1000 years)
Power input (kWh/kg)
2.0-5.0 kWh/kg
Used to solidify sludge, maintain storage facilities, and operate monitoring systems.
Fuel input Type and quantity (kg)
Diesel fuel: 0.5 to 1.0 kg
Operation of heavy equipment required for hauling and facility construction.
CO₂ emissions other than combustion (kg)
0.1 to 0.2 kg
A chemical reaction byproduct of the sludge solidification process or cementation.
Amount of soil, water, and other resources changed (kg)
Sediment transport: 10-50 kg
Excavation work for the construction of a quarantine facility.
Input Item Name and Input Quantity Guideline
Cement, bitumen: 1.2-1.5 kg
Used to solidify radioactive sludge.
Glass matrix material: 0.2 to 0.5 kg
Used in the vitrification process for high-level waste.
Lead, stainless steel: 0.1 to 0.3 kg
container sealing and shielding materials.
Solvents and other process substances Name, amount used, circulation rate
Acid neutralizer (NaOH): 0.1-0.3 kg / circulation rate: 0%.
Used to neutralize sludge. After treatment, disposed of as waste liquid.
Wastes requiring special treatment Name and quantity standard
Waste block after solidification: 1.5 to 2.0 kg
Increased waste volume after sludge solidification.
Effluent from storage facilities: 0.05-0.1 kg
treatment of water used for cooling and management.
Process Overview
Sludge Recovery and Neutralization Treatment
Sludge is chemically stabilized with an acid neutralizer (e.g., NaOH).
Solidification Treatment
Cement or vitrification technology is used to safely solidify radioactive sludge.
Container encapsulation
Solidified materials are encapsulated in lead or stainless steel sealed containers to prevent secondary leakage.
Transfer to Long-Term Storage Facility
Completed waste is transferred to a geologic disposal facility or special storage facility.
Monitoring and Maintenance
Storage facilities are regularly monitored by a monitoring system and repair work is performed as needed.
Catalyst usage and degradation rate
Catalysts: not used in particular. Mainly chemical processing.
supplementary information
Long-term isolation facilities (geological disposal) must be maintained for several hundred years or more and require large amounts of energy for construction and operation. In addition, monitoring and cooling of the storage facility until it stabilizes is essential.
The above calculations do not include the power and costs required to maintain monitoring systems and storage facilities over a 1000-year period. Such long-term monitoring typically includes the following elements, which add up to annual electricity consumption
Power and resources for long-term monitoring
Annual electricity consumption:
Surveillance systems (sensors, cameras, remote communications): 10-50 kWh per year
Air conditioning and cooling systems: 100-200 kWh per year
Facility lighting and security: 20-100 kWh per year
1000 years of accumulated electricity consumption:
The entire monitoring system is expected to consume 100,000 to 500,000 kWh.
Need for input fuel
Diesel fuel: used in emergency generators, 10-30 kg per year.
Upkeep and Facility Maintenance
Regular inspections and equipment replacement are required, and structural maintenance of isolation facilities involves the use of additional steel and cement.
Measures for long-term monitoring and maintenance
Renewable Energy: Some facilities have installed solar and wind power to reduce electricity demand.
Autonomous monitoring systems: implementing power-efficient IoT systems and automated monitoring technologies.
This long-term power consumption is often not included in the initial facility design, which can result in huge energy costs over a 1,000-year accumulation.
Glass matrix material for cement solidification
3-5 kWh/kg: Melting process (high-temperature furnace used for glass formation)
Input fuel
Natural gas: 0.5-1.0 kg/kg (fuel for melting furnaces)
Coke: sometimes used in part as a supplemental fuel
CO2 emissions other than combustion
0.1-0.3 kg CO₂/kg: gas derived from carbonates (e.g. from use of sodium carbonate)
Amount of change to resources such as soil and water
Silica sand: 1.1-1.3 kg
Borates (e.g. borax): 0.05-0.1 kg
Alumina (Al₂O₃): 0.05-0.2 kg
Input materials (names and approximate amounts)
Silica sand (SiO₂): 1.1-1.3 kg
Sodium carbonate (Na₂CO₃): 0.2-0.3 kg
Borax (Na₂B₄O₇): 0.1 kg
Solvents and process substances
Water: 0.5-0.8 kg (for washing and mixing)
Circulation rate: 90% or more reusable
catalyst
None (generally no catalysts are used, but organic additives are used in special cases)
Waste requiring special treatment
Glass waste and dust: 0.01-0.05 kg (must be reused or discarded)
Wastewater: 0.1-0.2 kg (requires neutralization treatment)
In this process, glass materials are melted at high temperatures and a matrix is formed by adding borax and sodium carbonate. These materials are used to solidify and stabilize waste and play a particularly important role in the treatment of radioactive waste.
Rare earth concentrates
Input power
0.5-1.5 kWh/kg: used in ore crushing and grinding and beneficiation equipment
Input fuel
Diesel fuel: 0.8-1.2 kg/kg (mining equipment and truck operations)
Fuel for power supply: may rely on coal or natural gas generation
CO₂ emissions other than combustion
0.02-0.05 kg CO₂/kg: from chemical use
Amount of change to resources such as soil and water
Waste rock and tailings: 5-10 kg (impurities in ore)
Process water: 3-5 kg (for washing and flotation processes)
Input materials (names and approximate amounts)
Ores (bastnaesite, monazite, xenotime, etc.)
5-7 kg (large amount of ore required due to rare-earth content of a few percent)
Flotation chemicals
Xanthate sorbent: 0.1-0.2 kg
Lime: 0.05-0.1 kg (for pH adjustment)
Solvents and other process substances
Water: 3-5 kg (for washing and flotation processes)
Circulation rate: 80-90% (reuse rate)
Catalyst Usage
Catalysts are generally not required.
However, acids and alkalis may be used to accelerate some chemical reactions during the treatment process.
Waste requiring special treatment
Tailings (may contain radioactive material): 10-15 kg
Contains thorium and uranium in monazite and other ores.
Wastewater: 1-3 kg (chemicals need to be removed)
In this process, the ore is crushed to obtain concentrates using beneficiation or flotation methods. In the flotation process, xanthates and foaming agents are used to efficiently separate the minerals. The resulting concentrates proceed to the rare-earth refining and extraction process.
Yttria 1kg
Input power
3-5 kWh/kg: Used in each treatment process such as solvent extraction, filtration and calcination.
Input fuel
Natural gas: 1.5-2 kg/kg (heat source in high temperature treatment and calcination processes).
Diesel: 0.2-0.3 kg (if used for material transport).
CO₂ emissions other than combustion
0.05-0.1 kg CO₂/kg: from chemical reactions and process water evaporation.
Amount of change to resources such as soil and water
Wastewater: 3-5 kg (emissions from solvent regeneration).
Solid waste (sludge): 0.5-1.2 kg (residue after solvent extraction).
Input materials (names and approximate amounts)
Rare earth concentrates (containing monazite and xenotime):
6-8 kg (yttrium content in concentrate is about 10-15%).
Sulfuric acid: 0.8-1.2 kg (used to dissolve rare earths).
Oxidant (hydrogen peroxide): 0.1-0.15 kg (to increase extraction efficiency).
Soda ash (sodium carbonate): 0.3-0.5 kg (pH adjusted).
Solvents and other process substances
D2EHPA (phosphate extractant): 0.05-0.08 kg (used in circulation at 80-90% extraction efficiency).
Kerosene (organic solvent): 0.4-0.5 kg (used as a medium for solvent extraction).
Circulation rate: 80-90%.
Catalyst Usage
No catalyst is required, but an alkaline agent is used for pH adjustment. Catalyst degradation does not occur.
Waste requiring special treatment
Sulfuric acid effluent: 2-3 kg (needs to be neutralized).
Solid residue (possibly contaminated with thorium/uranium): 1-2 kg.
In this process, rare-earth concentrates are dissolved in sulfuric acid and separated using organic extractants such as D2EHPA. Calcination is then used to obtain a stable oxide (yttria, Y₂O₃). pH adjustment to maintain the acid/alkaline balance is important, as is proper treatment of wastewater and waste.
Extract radioactive materials from 1 kg of rare earth tailings and process radioactive sludge
Input power
2-4 kWh/kg: required for solvent extraction, filtration, drying, and stabilization of radioactive sludge.
Input fuel
Natural gas: 1.5 kg/kg (used in drying and firing processes).
Diesel: 0.1-0.2 kg/kg (if used for waste transport or process operation).
CO₂ emissions other than combustion
0.05-0.1 kg CO₂/kg: indirect emissions from chemical reactions and solvent use.
Impact on resources such as earth, rocks, and water
Wastewater (including radioactive materials): 2-4 kg
Solid residues (residues not contained in radioactive waste): 0.5-1 kg
input
Tailings: 1 kg (tailings to be processed).
Sulfuric acid or hydrochloric acid: 0.5-1 kg (for elution of radioactive materials).
Sodium carbonate: 0.2-0.3 kg (for pH adjustment).
Organic solvent (e.g. D2EHPA): 0.05 kg (for extraction of radioactive components).
Coagulant: 0.1-0.15 kg (improves sludge generation efficiency).
Amount of radioactive sludge generated
Radioactive sludge: 0.2-0.4 kg/kg (thorium, uranium and other radioactive components in tailings).
Solvents and circulation rates
D2EHPA (phosphate extractant): 0.05 kg (80-90% circulation)
Kerosene: 0.2 kg (used as organic solvent, 90% circulation).
Waste requiring treatment
Effluent after neutralization: 1-2 kg (must be treated before discharge to the environment).
Radioactive sludge: 0.2-0.4 kg (requires long-term storage).
In this process, radioactive components are chemically eluted from the tailings and separated by solvent extraction. The extracted radioactive components are stabilized as sludge and finally treated as radioactive waste. After neutralization, the liquid waste is treated in a manner that conforms to safety standards.
Ceria (CeO₂)
Power input (kWh/kg)
2-5 kWh/kg: required for treatment processes such as dissolution, filtration and calcination.
Fuel input Type and quantity (kg/kg)
Natural gas: 0.5-1 kg (drying and firing process).
Diesel fuel: 0.1-0.2 kg (used for transportation and operation of some equipment).
CO₂ emissions other than combustion (kg CO₂/kg)
0.05-0.1 kg: Occurs as a byproduct of chemical reactions.
Impact on soil and water resources (kg/kg)
Wastewater: 3-5 kg (including drainage after acid treatment).
Solid waste: 1-1.5 kg (unused residual components and impurities).
Input Item Name and Input Quantity
Rare earth concentrates: 2-3 kg
Cerium-bearing concentrates (Ce content 15-30%).
Sulfuric acid: 1.0-1.5 kg
Used in acid dissolution process.
Sodium hydroxide: 0.3-0.5 kg
Used in the neutralization process.
Ammonium oxide: 0.1 kg
when used in the ion exchange process.
Solvent/process substance usage and circulation rate
D2EHPA (phosphate extractant): 0.05 kg (90% circulation)
Organic solvent (e.g. kerosene): 0.2 kg (85-90% circulation)
Catalyst use and degradation rate
None: Catalysts are generally not used in ceria production.
Waste requiring special treatment
Acid waste: 2-3 kg
Needs to be neutralized and treated.
Solid residues (impurities): 1.0 kg
Process Overview
Acid Dissolution: concentrates are dissolved in sulfuric acid to produce a liquid containing cerium.
Solvent extraction: cerium is separated from other rare earths using D2EHPA.
Neutralization and filtration: remove impurities and filter.
calcination: the final product is obtained as cerium oxide (CeO₂).
Ammonium oxide (NH₄NO₃)
Power input (kWh/kg)
0.5-1.5 kWh/kg: in-process compaction or mixing process.
Fuel input Type and quantity (kg/kg)
Natural gas: 0.2-0.5 kg (used for reaction heating and steam generation).
CO₂ emissions other than combustion (kg CO₂/kg)
0.1-0.2 kg: components of side reactions and exhaust gases.
Impact on soil and water resources (kg/kg)
Water: 1-2 kg (for cooling and cleaning).
Solid waste: 0.1-0.2 kg (e.g., impurity residues).
Input Item Name and Input Quantity Guideline
Nitric acid (HNO₃): 0.8-1.0 kg
reacts with ammonium to produce ammonium nitrate.
Ammonia (NH₃): 0.2-0.3 kg
Ammonium oxide is produced by reaction with nitric acid.
Solvent/process substance usage and circulation rate
Deionized water: 0.5-1.0 kg (used for washing, 70-80% circulation).
Catalyst use and degradation rate
No catalyst is used: no catalyst is used in the normal process.
Waste requiring special treatment
Wastewater (including nitric acid residue): 0.5-1.0 kg
Needs to be neutralized.
Gas emissions: 0.1-0.2 kg (trace emissions of nitrogen dioxide and ammonia).
Process Overview
Mixing reaction: nitric acid and ammonia react to produce ammonium nitrate.
Filtration and cooling: the solution is filtered and solid ammonium oxide is precipitated.
Drying and granulation: the final product is dried and granulated or powdered.
In this production process, most of the energy consumption is concentrated in the heating and drying processes. The treatment of wastewater and gases as byproducts is also an important environmental management issue.
Lanthanum oxide (La₂O₃)
Power input (kWh/kg)
4-8 kWh/kg: used for separation process, heating and drying.
Fuel input Type and quantity (kg/kg)
Natural gas or fuel oil: 0.5-0.8 kg (used for furnace heating process).
CO₂ emissions other than combustion (kg CO₂/kg)
0.05-0.2 kg: emissions from chemical reactions and by-products.
Impact on soil and water resources (kg/kg)
Water: 10-15 kg (for extraction, washing and cooling).
Waste (tailings and sludge): 2-4 kg (impurity residue after processing).
Input Item Name and Input Quantity Guideline
Rare earth concentrates: 4-6 kg (assuming 10-30% La₂O₃ content).
Acid (sulfuric or hydrochloric acid): 1-2 kg (removal of impurities and elution of lanthanum).
Alkali (sodium carbonate or sodium hydroxide): 0.5-1 kg (acid neutralization and precipitation promotion).
Solvent/process substance usage and circulation rate
D2EHPA (diethylhexyl phosphate): 0.1-0.2 kg, 80-90% circulation.
Organic extractants for the separation of lanthanum from other rare earths.
Catalyst use and degradation rate
Catalysts are not usually used.
Waste requiring special treatment
Acid sludge: 0.5-1 kg (acid and impurities after use).
Tailings residue: 2-3 kg (inorganic residue after rare earth separation).
Process Overview
Dissolution and Leaching: Rare earth concentrates are dissolved in acid to dissolve each element.
Extraction and separation: lanthanum was separated by solvent extraction using D2EHPA.
Precipitation and calcination: precipitate is produced and finally lanthanum oxide is obtained by calcination.
Because this process uses large amounts of acids and solvents, environmental measures are important. In addition, energy consumption is particularly concentrated in the calcination process, and disposal of waste acid and sludge is essential.
Neodymium metal (Nd)
Inputs and energy required to produce neodymium metal (Nd)
Power input (kWh/kg)
30-50 kWh:
Electrolytic or reduction process for reducing neodymium from oxides.
Fuel input (kg/kg)
Natural gas: 0.5-0.8 kg (furnace heating).
CO₂ emissions other than combustion (kg CO₂/kg)
0.1-0.3 kg: generated by chemical reactions during impurity removal.
Content and input in neodymium concentrate
Average content: 20-25% neodymium oxide (Nd₂O₃) in concentrate.
Input: 4-5 kg of concentrate is needed to obtain 1 kg of neodymium metal.
Acid (hydrochloric or sulfuric acid): 2-3 kg (leaching of rare earth elements).
Calcium metal: 0.5-0.7 kg (used to reduce neodymium metal).
Potassium chloride (KCl): 0.8-1.2 kg (used for molten salt electrolysis).
Solvents and other process substances Usage and circulation rate
Molten salt electrolyte (KCl-NaCl system): 1.0-1.5 kg, 90% circulation.
Catalyst use and degradation rate
No catalyst is used, but the calcium used for reduction is consumed.
Waste requiring special treatment
Acidic effluent: 1-2 kg (effluent after acid leaching).
Tailings residue: 2-3 kg (impurities after beneficiation).
Process Overview
Acid leaching: Leaching of rare earths from concentrates with hydrochloric or sulfuric acid.
Solvent Extraction: Neodymium is separated with D2EHPA or other solvents.
Reduction process: reduction of metallic neodymium from neodymium oxide (Nd₂O₃) using calcium metal.
Molten Salt Electrolysis: Using KCl-NaCl molten salts to obtain purified neodymium metal.
The process is energy intensive and requires a lot of electricity, especially for electrolysis and reduction. It is also characterized by the use of calcium as a reducing agent, making proper treatment of waste acid and tailings important.
calcium metal
Power input (kWh/kg)
8-12 kWh:
Used for electrolytic processes.
Fuel input Type and quantity (kg/kg)
Natural gas: 0.4-0.6 kg (used to heat the furnace).
CO₂ emissions other than combustion (kg CO₂/kg)
0.2-0.4 kg:
Arising from chemical transformation of raw materials.
Impact on resources such as soil and water (kg/kg)
Water usage: 10-15 kg (cooling and cleaning processes).
Slag as by-product: 0.5-1 kg (after decomposition of calcium chloride).
Input Item Name and Input Quantity
Calcium chloride (CaCl₂): 2.0-2.5 kg (main source of calcium).
Electrolyte (sodium chloride, NaCl): 0.8-1.0 kg (as an aid to electrolysis).
Graphite: 0.05-0.1 kg (cathode material).
Solvents and other process substances Usage and circulation rate
Molten salt (CaCl₂-NaCl system): 1.5-2.0 kg, 90% circulation.
Catalyst use and degradation rate
No catalyst (no catalyst is used as it is produced by direct electrolysis).
Waste requiring special treatment Name and quantity standard
Slag (impurity content): 0.5-1.0 kg.
Electrolysis effluent: 1.5-2.0 kg (non-circulating minutes).
Process Overview
Raw Material Preparation: Prepare calcium chloride (CaCl₂) as a raw material.
Molten salt electrolysis: CaCl₂-NaCl-based molten salt electrolysis is used to deposit metallic calcium at the cathode.
Cooling and recovery: the calcium metal produced is cooled and separated from the slag.
This process consumes a relatively large amount of electricity, mainly due to electrolysis. In addition, some of the slag and electrolyte are reused, but some waste must be disposed of.
metal-plaseodymium
Power input (kWh/kg)
15-20 kWh:
Used for electrorefining or reduction processes.
Fuel input Type and quantity (kg/kg)
Natural gas or coke: 0.3-0.5 kg (used to heat the reduction furnace).
CO₂ emissions other than combustion (kg CO₂/kg)
0.1-0.3 kg:
generated by concentrate processing and reduction reactions.
Change to resources such as soil and water (kg/kg)
Water usage: 10-15 kg (used for washing and cooling).
Effluent: 1-1.5 kg (effluent after removal of impurities).
Input Item Name and Input Quantity
Rare earth concentrates containing praseodymium: 4-6 kg (depending on content).
Calcium chloride (CaCl₂): 1.5-2.0 kg (electrolyte for reduction reaction).
Praseodymium oxide (Pr₂O₃): 1.1-1.2 kg (oxide for reduction).
Solvents and other process substances Usage and circulation rate
CaCl₂-NaCl molten salt: 1.5-2.0 kg, 90% circulation.
Catalyst use and degradation rate
No catalyst (produced by direct reduction or electrolysis).
Wastes requiring special treatment Name and quantity standard
Calcium oxide (CaO): 0.8-1.2 kg (produced as a by-product).
Wastewater: 1-1.5 kg (as process wash water).
Process Overview
Reduction of praseodymium oxide: praseodymium oxide (Pr₂O₃) is taken from concentrate and reduced in CaCl₂ molten salt.
Electrolysis or substitution reaction with calcium: reduction of oxide to produce praseodymium metal.
Cooling and washing: Cool the product and remove the residue.
This process requires a lot of energy for the reduction reaction and refining process to extract praseodymium from rare-earth concentrates. In addition, since calcium salts are used, calcium oxide is produced as a byproduct.
Inputs and energy required to produce praseodymium metal (Pr)
Power input (kWh/kg)
20-30 kWh:
Reduction of praseodymium oxide to metal.
Fuel input (kg/kg)
Natural gas: 0.4-0.7 kg.
Content and input in praseodymium concentrate
Average content: 4-5% praseodymium oxide (Pr₆O₁₁) in concentrate.
Input: 20-25 kg of concentrate is needed to obtain 1 kg of praseodymium metal.
Process substances and by-products
Molten salt (CaCl₂): 1.5-2.0 kg used, 90% circulation.
Calcium oxide (CaO): 1-1.2 kg generated as a byproduct.
Wastewater: 1.5-2.0 kg Treatment required.
metallic samarium
Power input (kWh/kg)
50-70 kWh: solvent extraction and subsequent electrolytic reduction process (conversion of samarium oxide to samarium metal).
Fuel input (kg/kg)
Natural gas: 0.7-1.2 kg
(for furnace firing and heat treatment of oxides)
CO₂ emissions other than combustion (kg CO₂/kg)
0.1-0.3 kg: emissions from side reactions and impurity removal processes.
Samarium content and input in concentrate
Average content: 1-2% samarium oxide (Sm₂O₃) in concentrate.
Input concentrate: 50-100 kg of concentrate is required to obtain 1 kg of samarium metal.
input
Concentrate: 50-100 kg
Calcium oxide (CaO): 5-8 kg (as flux for reduction process)
Molten salt (LiCl or CaCl₂): 2-3 kg used, 90% circulation
Solvents and extractants
D2EHPA (diethylhexyl phosphate): 1.0-1.5 kg
Tributyl phosphate (TBP): 0.5-1.0 kg
Circulation rate: 90-95%.
Waste requiring special treatment
Waste acid: 2-4 kg
Insoluble sludge: 1.5-2.0 kg
Catalyst use and degradation
Acid catalyst (HNO₃): 0.2-0.5 kg Degradation rate: 1%/process cycle
Process Overview
Solvent extraction: stepwise extraction using D2EHPA or TBP is used to extract samarium oxide from rare-earth concentrates.
Reduction reaction: samarium oxide (Sm₂O₃) is reduced using calcium metal.
Purification and final process: After extraction, purity is improved by acid washing and heat treatment.
The process combines the separation of rare earths by solvent extraction with metal reduction and is designed to efficiently use the materials and energy required to produce samarium. Due to the low content in concentrates, inputs tend to be high.
Inputs and energy to obtain 1 kg of europium oxide (Eu₂O₃) from a rare earth concentrate
A separation process using multi-stage solvent extraction is commonly used to produce europium oxide. This process involves many chemical and energy inputs to separate europium from other rare-earth oxides at high purity. The following is a rough guide to the process.
- inputs and energy
Input power:
10-15 kWh/kg
Fuel input:.
Fuel oil or natural gas: 0.5-0.7 kg
CO₂ emissions other than combustion:
0.1-0.2 kg
Amount of change to resources such as earth, rocks, water, etc.:.
Liquid and solid waste: 2-3 kg
- details of inputs
| name | Suggested input amount |
| Rare earth concentrates | 30-50 kg (0.2% Eu₂O₃ content) |
| Sulfuric acid (H₂SO₄) | 2-3 kg |
| Sodium carbonate (Na₂CO₃) | 1-2 kg |
- process substances such as solvents
| name | amount used | Cycloparametric rate |
| Ion exchange resin or organic extractant (e.g., D2EHPA) | 0.2-0.3 kg | 95-98% of |
| Dilute nitric acid (HNO₃) | 1-1.5 kg | 70-80% of |
- waste requiring special treatment
| name | Quantity standard |
| acidic waste liquid | 3-4 kg |
| Solid Residue | 0.5-1 kg |
- process overview
Acid leaching process:
concentrate is dissolved in sulfuric or hydrochloric acid to produce a solution containing europium.
Multi-stage solvent extraction:
Europium is separated from other rare-earth elements using a solvent such as D2EHPA (di(2-ethylhexyl)phosphate).
Concentration and precipitation:
precipitate europium with sodium carbonate to obtain europium oxide.
Waste Disposal:
Properly dispose of acidic effluents and solid residues.
The process is multi-stage and high energy intensive. Efficiency is particularly important for the recovery of elements such as europium, which is present in small amounts, and requires highly efficient use of concentrates and optimization of recirculation.
Gadolinium oxide (Gd₂O₃)
The production of gadolinium oxide (Gd₂O₃) involves a multi-stage solvent extraction process from other rare earth elements. This process requires a lot of resources and energy to obtain a high purity product, and the elemental content of the concentrate is also a consideration.
Input power (kwh/kg)
Input power: 12-20 kWh/kg
- input fuel
Natural gas or fuel oil: 0.4-0.6 kg
3 CO₂ emissions other than combustion
CO₂ emissions: 0.15-0.25 kg
- changes to resources such as earth, rocks, and water
Liquid waste and solid residues: 2-4 kg
- standard for inputting materials
| name | Suggested input amount |
| Rare earth concentrates | 30-40 kg (1% gadolinium content) |
| Sulfuric acid (H₂SO₄) | 2-3 kg |
| Sodium carbonate (Na₂CO₃) | 1-2 kg |
- process substances such as solvents
| name | amount used | Cycloparametric rate |
| D2EHPA (di(2-ethylhexyl)phosphate) | 0.2-0.3 kg | 95-98% of |
| Dilute nitric acid (HNO₃) | 1-1.5 kg | 70-80% of |
- waste requiring special treatment
| name | Approximate quantity |
| acidic waste liquid | 3-4 kg |
| Solid Residue | 0.5-1.5 kg |
Process Overview
Acid Leaching
Treat the concentrate with sulfuric or hydrochloric acid to create a solution containing gadolinium.
Multi-stage solvent extraction
Separate gadolinium from other rare earth elements using D2EHPA or ion exchange resins.
Precipitation and calcination
After precipitation with sodium carbonate, gadolinium oxide (Gd₂O₃) is obtained by calcination at high temperature.
Waste Disposal
Properly dispose of acidic effluents and solid residues generated by the process.
The process is energy intensive and requires multiple stages of extraction and calcination. Due to the low gadolinium content, an efficient process design is required. In addition, the cost and environmental impact can be reduced by increasing the circulation rate of the solvent used.
Terbium metal (Tb)
Terbium metal is typically produced through a multi-stage solvent extraction process from rare-earth concentrates. Because of the low content of terbium, large volumes of concentrates must be processed. Phosphate extractants and ion exchange are also used for extraction.
Input power (kWh/kg)
Power input: 30-40 kWh
- input fuel
Natural gas or fuel oil: 0.5-0.8 kg
3 CO₂ emissions other than combustion
CO₂ emissions: 0.3-0.5 kg
- amount of resource change to soil and water
Liquid waste and solid residues: 3-5 kg
- standard for inputting materials
| name | Suggested input amount |
| Rare earth concentrates | 40-50 kg (terbium content 0.1-0.2%) |
| Sulfuric acid (H₂SO₄) | 2-3 kg |
| Sodium carbonate (Na₂CO₃) | 1.5-2 kg |
- solvents and process substances
| name | amount used | Cycloparametric rate |
| D2EHPA (di(2-ethylhexyl)phosphate) | 0.3-0.5 kg | 95-98% of |
| Dilute nitric acid (HNO₃) | 1-1.2 kg | 75-85% of |
- waste requiring special treatment
| name | Quantity standard |
| acidic waste liquid | 4-5 kg |
| Solid Residue | 1-2 kg |
Process Overview
Acid Leaching Treatment
Rare earth concentrates are treated with acid to produce a solution containing terbium.
Multi-Stage Solvent Extraction
Use phosphate extractants (such as D2EHPA) to separate terbium from other rare earths.
Precipitation and Reduction
After precipitation with sodium carbonate, terbium oxide is obtained by calcination at high temperature. Finally, it is converted to terbium metal by calcium reduction.
Waste Disposal
Some of the acids and solvents used will be reused, but unused portions will be disposed of properly.
Dysprosium metal (Dy)
Dysprosium is widely used as an important magnetic material among the rare earth elements and is usually obtained from concentrates through a multi-stage solvent extraction and reduction process.
Input power (kWh/kg)
Power input: 35-45 kWh
- input fuel
Natural gas or fuel oil: 0.6-0.9 kg
3 CO₂ emissions other than combustion
CO₂ emissions: 0.4-0.6 kg
- the amount of change to resources such as earth, rocks, and water
Liquid waste and solid residues: 5-6 kg
- standard for inputting materials
| name | Suggested input amount |
| Rare earth concentrates | 50-60 kg (Dy content 0.1-0.15%) |
| Sulfuric acid (H₂SO₄) | 3-4 kg |
| Sodium carbonate (Na₂CO₃) | 2 kg |
- solvents and process substances
| name | amount used | Cycloparametric rate |
| D2EHPA (di(2-ethylhexyl)phosphate) | 0.3-0.4 kg | 95-98% of |
| Dilute nitric acid (HNO₃) | 1.5-2 kg | 80-90% of |
- waste requiring special treatment
| name | Approximate quantity |
| acidic waste liquid | 5-6 kg |
| Solid Residue | 2-3 kg |
Process Overview
Acid Leaching Process
Rare earth concentrates are treated with sulfuric acid or other chemicals to produce a solution containing dysprosium.
Multi-stage solvent extraction
Dysprosium is separated from other rare earths using extractants such as D2EHPA.
Precipitation and calcination
The precipitate is formed with sodium carbonate and calcined at high temperature to obtain dysprosium oxide.
Reduction Process
Oxides are reduced with calcium to obtain the metal dysprosium.
Waste Management
Some acidic effluents and solid residues are recycled, but must be disposed of properly.
The process requires the processing of large volumes of concentrates due to the very low dysprosium content in the rare-earth concentrates. It also ensures a high circulation rate of solvents and reductants, thereby reducing costs and environmental impact.
Erbium oxide (Er₂O₃)
Erbium oxide is mainly used in fluorescent materials and glass additives and is separated from other rare earths through multi-stage solvent extraction.
Input power (kWh/kg)
Electricity: 30-40 kWh
- input fuel
Fuel oil or natural gas: 0.5-0.8 kg
3 CO₂ emissions other than combustion
CO₂ emissions: 0.4-0.6 kg
- the amount of change to resources such as earth, rocks, and water
Liquid waste and solid residues: 4-5 kg
- standard for inputting materials
| name | Suggested input amount |
| Rare earth concentrates | 45-55 kg (Er content 0.2-0.3%) |
| Sulfuric acid (H₂SO₄) | 2-3 kg |
| Sodium hydroxide (NaOH) | 1.5 kg |
- solvents and process substances
| name | amount used | Cycloparametric rate |
| D2EHPA (di(2-ethylhexyl)phosphate) | 0.2-0.3 kg | 95-97% of |
| Dilute nitric acid (HNO₃) | 1-1.5 kg | 85-90% of |
- waste requiring special treatment
| name | Approximate quantity |
| acidic waste liquid | 4-5 kg |
| Solid Residue | 2-3 kg |
Process Overview
Acid Leaching Treatment
Rare earth concentrates are treated with sulfuric acid to produce a solution containing erbium oxide.
Multi-stage solvent extraction
Use an extractant such as D2EHPA to separate erbium from other rare earths.
Precipitation and calcination
Precipitation is formed with sodium hydroxide and calcined to obtain erbium oxide.
Waste Management
Waste management is neutralized and reused, and some is disposed of properly.
The process requires high throughput of concentrates and the extractant used is reused at a high recirculation rate, but requires treatment of acidic effluents. Energy management throughout the process is also important.
If the amount of concentrate required to obtain 1 kg of erbium (Er) from a rare earth concentrate is 100-300 kg, the input of each element reflecting the typical content of holmium (Ho), thulium (Tm), ytterbium (Yb) and lutetium (Lu) is as follows.
- typical content of each element in concentrate (reference values)
Hormium (Ho): 0.05-0.1%.
Thulium (Tm): 0.01-0.03%.
Ytterbium (Yb): 0.05-0.2%.
Lutetium (Lu): 0.005-0.02%.
- input of each element in 100-300 kg of concentrate
| chemical element | Content (approximate) | Input of 100 kg of concentrate (g) | Input of 300 kg of concentrate (g) |
| Hormium (Ho) | 0.05 to 0.1% (0.05 to 0.1% of the total) | 50-100g | 150-300g |
| Thulium (Tm) | 0.01-0.03 | 10-30g | 30-90g |
| Ytterbium (Yb) | 0.05-0.2 | 50 to 200g | 150-600g |
| Lutetium (Lu) | 0.005 to 0.02% (0.005 to 0.02%) | 5-20g | 15-60g |
Supplementary Explanation
Variation in content: The content of these rare earth elements varies from deposit to deposit, especially the heavy rare earths (holmium, thulium, ytterbium, and lutetium), which have very low concentrations.
need for high purity products: to obtain the final oxide or metal product, these elements are further separated and purified by solvent extraction or ion exchange methods.
Energy Cost Considerations: Due to the low content of rare elements, multi-step extraction to extract them requires high energy and solvent consumption.
Thus, although the other heavy rare earth elements obtained in the process of obtaining 1 kg of erbium are very small in quantity, precise separation is required, so input and energy management at each stage is critical.
The following table shows the input of concentrates to obtain 1 kg of each element (erbium, holmium, thulium, ytterbium, and lutetium). These values are calculated from typical concentrations of rare earths in rare earth ores.
- general content of each element (typical example)
Hormium (Ho): 0.05-0.1%.
Thulium (Tm): 0.01-0.03%.
Ytterbium (Yb): 0.05-0.2%.
Lutetium (Lu): 0.005-0.02%.
Erbium (Er): 0.05-0.2%.
- example of calculation of concentrate input to obtain 1 kg of metal
| chemical element | Content (Typical) | Required amount of concentrate (approximate) |
| Hormium (Ho) | 0.05 to 0.1% (0.05 to 0.1% of the total) | 1,000 to 2,000 kg |
| Thulium (Tm) | 0.01-0.03 | 3,300 to 10,000 kg |
| Ytterbium (Yb) | 0.05-0.2 | 500 to 2,000 kg |
| Lutetium (Lu) | 0.005 to 0.02% (0.005 to 0.02%) | 5,000-20,000 kg |
| Erbium (Er) | 0.05-0.2 | 500 to 2,000 kg |
- calculation method
Metal weight (1 kg) ÷ content = required concentrate
e.g.: Thulium:
1 kg ÷ 0.01 = 10,000 kg
Because of the low concentration of each element, a very large amount of concentrate must be processed to obtain a particular metal. The low concentrations of elements such as lutetium and thulium, in particular, result in large inputs.
- points to note
Multi-step extraction process: these elements are generally obtained in a multi-step solvent extraction or separation process.
Energy Consumption: The energy and cost of processing is significant and must be managed at each stage.
Generation of by-products: Since other rare earths are often obtained simultaneously in this process, optimal processing is required for overall efficiency.
Thus, the recovery of rare earths requires an extremely precise process, and the concentrate input per kg of each metal can be very high.
thorium (Th)
- thorium recovery overview
Thorium is obtained as a byproduct from rare earth ores such as monazite and bastnaesite. These ores contain about 0.1-12% thorium. Acid leaching and solvent extraction methods are commonly used to extract thorium, which also requires the disposal of radioactive waste.
- inputs and approximate energy required to obtain 1 kg of thorium
| (data) item | Value (approximate) |
| Input power | 3-5 kWh/kg |
| Input fuel | 0.2-0.5 kg natural gas or coal |
| Other than combustion, CO₂ | 0.5 to 1.0 kg |
| Amount of soil and water change | 5,000 to 10,000 kg |
| input | 20-100 kg concentrate (depending on content) |
| solvent | Nitric acid, sulfuric acid 2-5 kg (circulation rate: 80%) |
| catalyst | Not used (or 0.01 to 0.1 kg of special adsorbent) |
| Waste requiring special treatment | Radioactive sludge 0.5 to 2 kg |
- process details
Leaching of ore concentrates
Monazite and other concentrates are dissolved in nitric or sulfuric acid to separate thorium.
Multi-stage solvent extraction
Thorium is extracted with a solvent (mainly TBP: tributyl phosphate) to separate it from other rare earths.
Precipitation or recrystallization
Separate and purify as thorium nitrate or thorium oxide (ThO₂).
Waste Disposal
Radioactive sludge generated during the extraction process needs to be treated and stored for a long period of time.
- example of input calculation
Thorium content: typical content of monazite is 6-12%.
Required concentrate to obtain 1 kg of thorium:
1 kg ÷ 0.1 (10%) = 10 kg
1 kg ÷ 0.06 (6%) = 16.7 kg
- points to note
Radioactive Waste Management: Because of the radioactive nature of the material, the waste generated during the extraction process is strictly controlled.
Process optimization: increased solvent reuse is required.
Thus, thorium production involves radioactive materials, so not only energy costs but also waste disposal is an important factor.
thorium concentrate
- outline of thorium concentrate acquisition
Thorium is found primarily in monazite and bastnaesite and is obtained as a byproduct along with rare earths. Thorium content in monazite ranges from 6 to 12%, but beneficiation, flotation, and the use of acids are required to convert the ore from mining to concentrate.
- inputs and approximate energy required to obtain 1 kg of thorium concentrate
| (data) item | Value (approximate) |
| Input power | 0.5 to 1.5 kWh/kg |
| Input fuel | Diesel oil/diesel 0.2 to 0.4 kg/kg |
| Other than combustion, CO₂ | 0.1 to 0.2 kg |
| Amount of change in soil, rocks, water, etc. | 1,000-2,500 kg (mined) |
| input | Monazite or bastnaesite ore 15-20 kg |
| Process Acids | Sulfuric acid, nitric acid, etc. 1-3 kg (circulation rate 70-80%) |
| Solvents and flotation agents | Fatty acid-based foaming agent 0.1-0.2 kg |
| Catalyst or adsorbent | not used |
| Waste requiring special treatment | 2-5 kg tailings, 1-2 kg acidic liquid waste |
- process details
Mining:
Open pit or underground mining of monazite and bastnaesite, which contain thorium. The content per ton of ore is about 1-10%, and concentrates are obtained by flotation or other methods.
Beneficiation and Flotation:
Mined ore is crushed and ground to concentrate thorium and rare earths using a fatty acid-based flotation agent. Unnecessary tailings are removed here.
Acid Treatment:
concentrates are acid treated with sulfuric acid or
nitric acid to dissolve thorium and rare earths. Some of the waste liquid is recovered and circulated for reuse.
Waste Disposal:
tailings and acidic effluents are generated as byproducts of the beneficiation process and are properly managed.
- consideration of content
The thorium content in monazite is approximately 6-12%, so roughly 15-20 kg of monazite is required to obtain 1 kg of thorium concentrate. Management of tailings and waste liquids is essential, and in particular, increasing the rate of acid circulation is a key to improving efficiency.
Thus, thorium extraction and mineralization involves high energy consumption and waste management, requiring optimization of methods to reduce environmental impact.
Uranium oxide (U₃O₈)
- outline
Uranium oxide (U₃O₈) is an important compound obtained from uranium concentrates (yellowcake) and is a fundamental material in the nuclear fuel cycle. The production process consists of refining and oxidation of the concentrate, using nitric acid and organic extractants.
- input materials, energy guideline
| (data) item | Value (approximate) |
| Input power | 5~7 kWh/kg |
| Input fuel | Natural gas 0.3-0.5 kg/kg |
| CO₂ emissions other than combustion | 0.1 to 0.2 kg |
| Amount of change in soil, rocks, water, etc. | Concentrate 2.5-3.0 kg/kg Uranium oxide |
| Input Item Name | Uranium concentrate (yellowcake) 2.5-3.0 kg |
| Process Solvents | 1.0-2.0 kg nitric acid, 0.1 kg tributyl phosphate |
| Circulation rate (solvent) | 80-90% of the total |
| Catalyst or adsorbent | none in particular |
| Waste requiring special treatment | Radioactive sludge 0.5-0.8 kg |
- manufacturing process details
Extraction and Refining of Concentrates
After uranium ore is mined, it is concentrated as uranium concentrate (yellowcake). This concentrate contains about 70-80% U₃O₈.
Acid Dissolution and Extraction Processes
Uranium concentrates are dissolved in nitric acid to obtain uranyl nitrate. Purification is then performed by solvent extraction using tributyl phosphoric acid (TBP).
Oxidation Treatment
The uranium solution after solvent extraction is calcined at high temperatures to convert it to uranium oxide (U₃O₈). Heating is typically in the range of 700-800°C.
Waste Disposal
Radioactive sludge and liquid waste generated by the process are strictly controlled and properly disposed of.
- consideration of content
The U₃O₈ content in uranium concentrates (yellowcake) is 70-80%, so 2.5-3.0 kg of concentrate is needed to obtain 1 kg of uranium oxide.
This manufacturing process is energy intensive and waste disposal is critical to minimize environmental impact. Solvent reuse is also key to improving efficiency.